Evaxion (EVAX)·Q3 2025 Earnings Summary
Evaxion Q3 2025 Earnings
Executive Summary
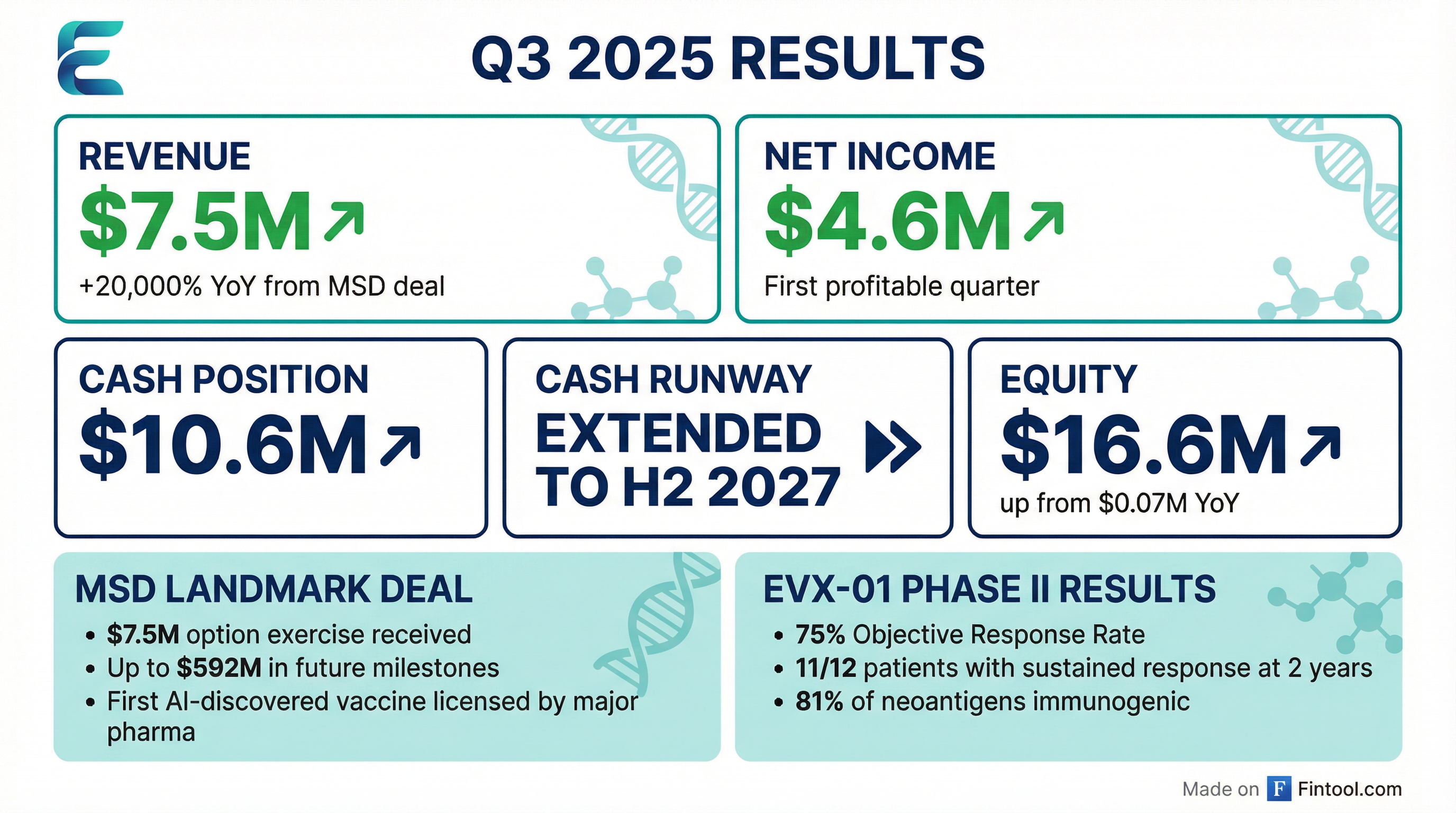
Evaxion delivered a transformative Q3 2025, reporting its first-ever profitable quarter driven by MSD's $7.5M option exercise on EVX-B3—marking the first AI-discovered vaccine candidate ever licensed by a major pharmaceutical company.
Key highlights:
- Revenue: $7.49M vs. $2.5M consensus (+200% beat)
- Net Income: $4.6M (first profitable quarter)
- Cash Runway: Extended to H2 2027
- EVX-01 Phase II: 75% objective response rate at 2 years
- New CEO: Dr. Helen Tayton-Martin appointed, effective November 24
Stock Reaction
Financial Results
Revenue & Profitability
*Values retrieved from S&P Global
Balance Sheet
*Values retrieved from S&P Global
Key drivers:
- $7.5M MSD option exercise fee drove Q3 revenue
- $1.3M net financial income from EIB debt-to-equity conversion at 89% premium
- Operating expenses at $4.47M, slightly below prior year
- Debt eliminated via EIB conversion in July
Beat/Miss History
What Went Well
1. MSD Landmark Partnership
"September 25th was a historical moment for Evaxion, with MSD exercising their option on EVX-B3. This was the first-ever in-licensing of an AI-discovered vaccine candidate by a major pharma company."
- $7.5M upfront payment received
- Up to $592M in future milestone potential
- All future EVX-B3 development costs borne by MSD
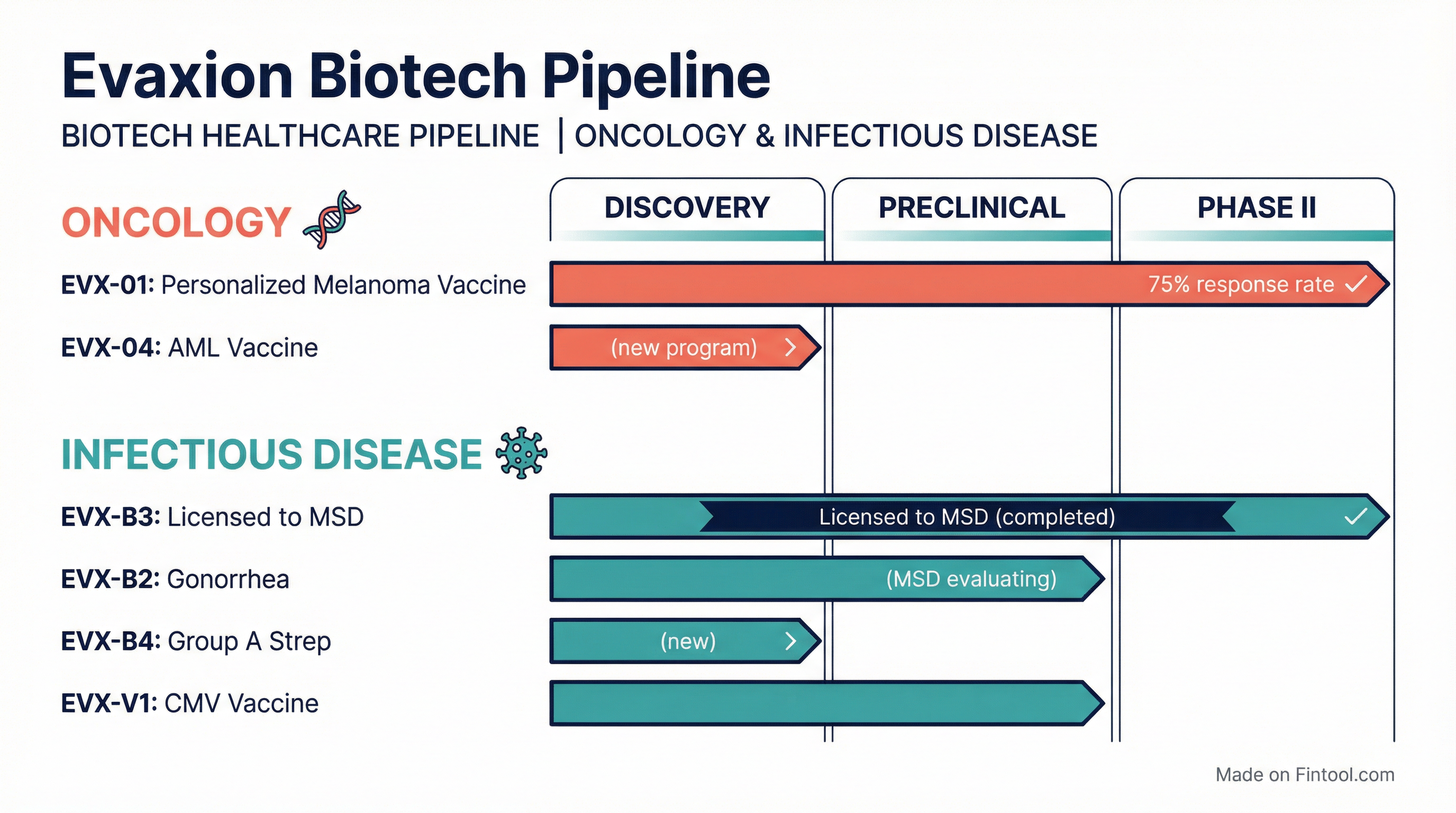
- EVX-B2 evaluation period extended to H1 2026
2. EVX-01 Phase II Clinical Success
"At the ESMO Congress, we reported a 75% objective overall response. We also saw that 11 out of 12 patients that responded had a sustained response at the two-year mark."
- 81% of neoantigens were immunogenic across patients
- 100% of patients showed neoantigen-specific T-cell response
- 34% conversion rate (patients deepening response)
- Durable T-cell responses sustained beyond dosing period
3. Pipeline Expansion
- EVX-04 added: Novel AML cancer vaccine targeting ERVs (endogenous retroviruses)
- Automated vaccine design module deployed, reducing design time and costs
What Went Wrong
1. Market Uncertainty Impact
"Market uncertainty affects deal climate... prolonging some discussions."
- Partnership discussions proceeding slower than hoped
- EVX-02 program discontinued (no active development)
2. Stock Underperformance
- Despite positive earnings, stock down 84% YTD
- Trading near 52-week lows despite operational progress
R&D Pipeline Update
Oncology Programs
Infectious Disease Programs
Management Commentary
On EVX-01 Partnership Outlook
"The questions are not about the quality of the data or the impact of the data, but more how can we move this forward together and find solutions for manufacturing and also for other indications."
On Business Development Pipeline
"We do have multiple dialogues ongoing... interest is across our R&D pipeline, but also centered around our capabilities for identifying novel targets."
On AI Platform Enhancement
"From data input to candidate generation, the process is now fully automated, ensuring optimal sequence and conformation of vaccine targets... speeds up vaccine development while reducing costs compared to traditional methods."
Q&A Highlights
EVX-01 Partnership Discussions (Soumit Roy, JonesTrading)
Q: What key questions are partners asking about EVX-01?
A: "We have moved from questions around quality of data, how does your platform work, to more, how can we apply your technology within perhaps other types of cancer indications... the strategy is that we will out-license it at the current development stage."
EVX-04 Target Differentiation (Soumit Roy, JonesTrading)
Q: How does EVX-04 differentiate from other AML targets like CD33, CD123?
A: "We look at genomic and transcriptomic data... in AML, there's a very high expression level of these endogenous retroviral sequences from the dark genome... This is an off-the-shelf approach where we will be able to use the same vaccine across different tumor profiles."
MSD EVX-B2 Timeline (RK, H.C. Wainwright)
Q: What's needed for MSD to decide on EVX-B2?
A: "MSD is currently evaluating the data that we have provided, and further, they are in the process of generating some confirmatory analysis... we expect that they will come back with an answer in the first half of next year."
AI Platform Monetization (RK, H.C. Wainwright)
Q: Can the new automated design module be licensed separately?
A: "We also do see an option of using this capability to support other companies in ensuring that what they select as their key antigens... can also be produced in a cost-effective way. I do see multiple options for monetizing on this new module."
Management Tone Evolution
Guidance & Outlook
Key Takeaways
-
Transformative quarter: First profitable quarter ever, driven by MSD's historic $7.5M option exercise on EVX-B3—the first AI-discovered vaccine licensed by major pharma
-
Clinical validation: EVX-01 Phase II showed 75% ORR at 2 years with durable responses, positioning for partnership discussions
-
Financial stability: Cash runway extended to H2 2027; debt eliminated via EIB conversion; equity strengthened to $16.6M
-
Leadership transition: New CEO Dr. Helen Tayton-Martin brings 30+ years biotech experience including Adaptimmune co-founding
-
Pipeline momentum: EVX-04 (AML) added, AI platform enhanced with automated design module, EVX-B2 MSD evaluation extended
-
Risks remain: Stock down 84% YTD despite operational progress; partnership timing uncertain amid market conditions