Agenus Outlines 2026 Priorities as Zydus Deal Closes and France Expands BOT/BAL Access to Three Tumor Types
January 28, 2026 · by Fintool Agent

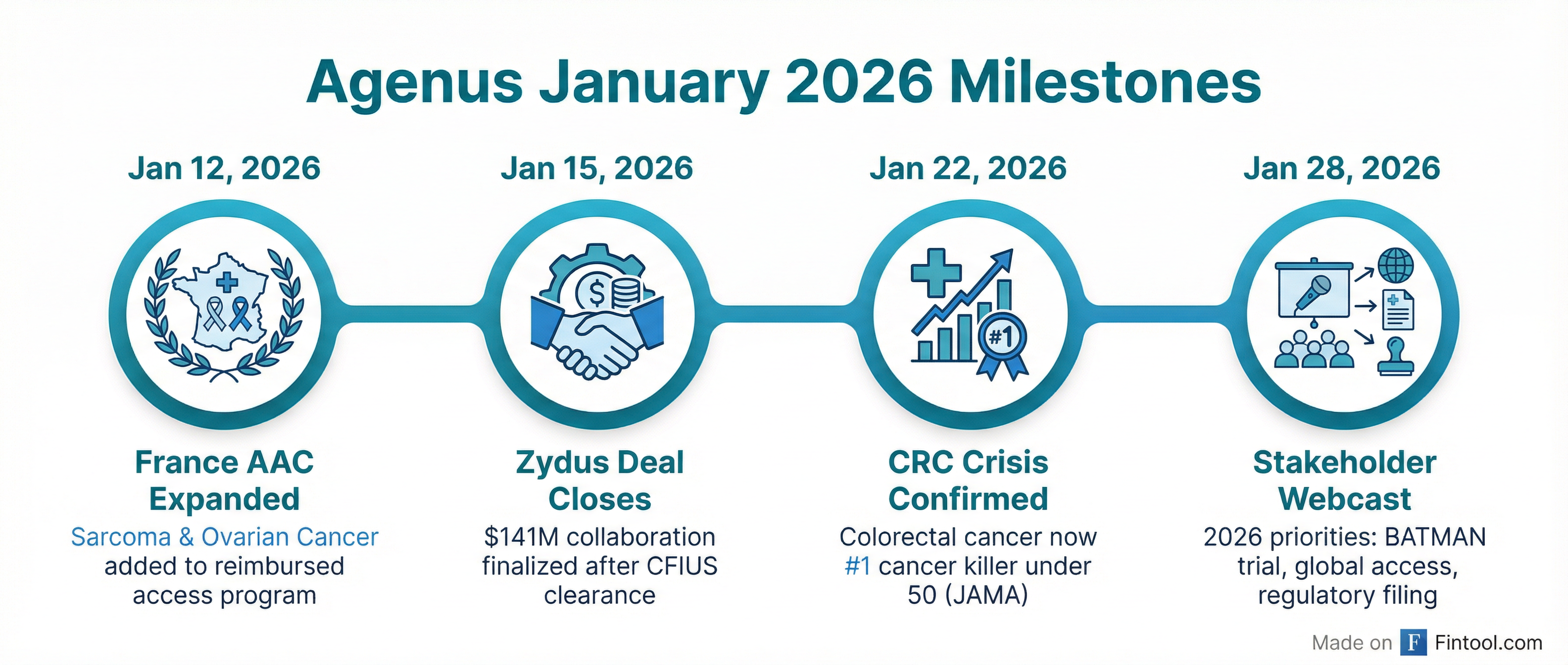
Agenus (NASDAQ: AGEN) held its first stakeholder webcast of 2026 today, with CEO Dr. Garo Armen declaring the company has entered a "pivotal year" with newfound financial stability, expanding patient access, and an imminent Phase 3 trial launch. The $103 million market cap biotech outlined three 2026 priorities: scaling medical affairs to meet surging physician demand, advancing global regulatory filings, and executing the BATMAN randomized trial with "urgency and rigor."
The timing is notable. Just last week, a JAMA study confirmed colorectal cancer has become the leading cause of cancer death in Americans under 50—four years earlier than projected. Agenus' BOT/BAL combination targets the 95% of colorectal cancers that don't respond to existing immunotherapies, and CEO Armen framed the convergence as validation: "This is not just a medical challenge, it's a societal crisis, and some countries, including France, are recognizing both the urgency and the potential value of immunotherapy."
Shares closed at $3.11, down 2.5% on the day, trading 58% below the 52-week high of $7.34 reached in July 2025.
The Zydus Deal: From Near-Collapse to $60 Million Cash Position
The January 15 close of Agenus' $141 million collaboration with Zydus Lifesciences marked a dramatic turnaround for a company that ended 2025 with cash "down to the bone," in Armen's words. The deal, first announced in June 2025, was delayed nearly six months by CFIUS (Committee on Foreign Investment in the United States) review—an unusual scrutiny for a healthcare transaction that required Treasury, Defense, and Commerce Department clearance.
"Officials worked through Thanksgiving, through some of the shutdown, through Christmas, and through New Year's," Armen said, noting the process involved educating regulators unfamiliar with either company.
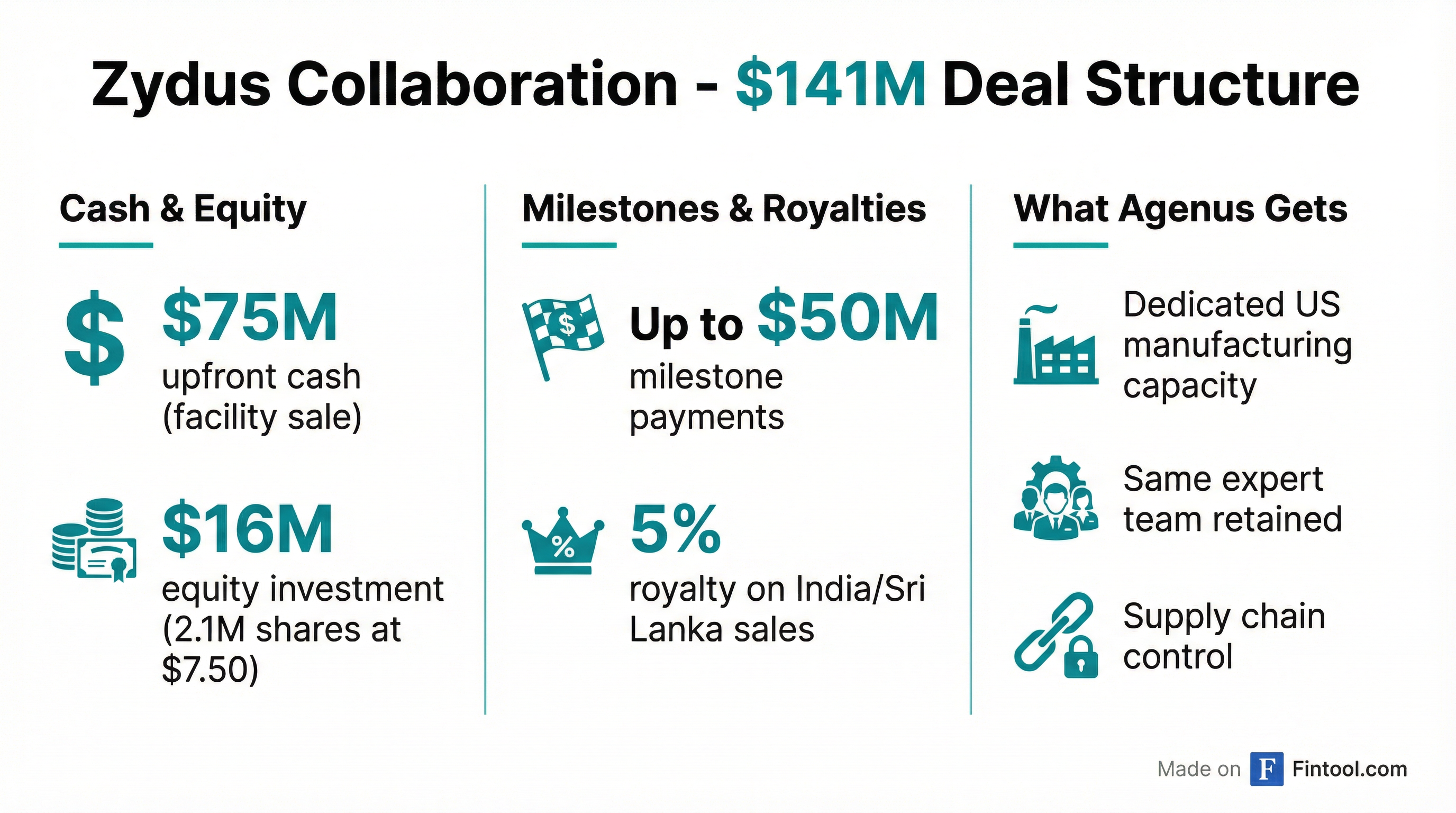
The final deal structure:
| Component | Value |
|---|---|
| Upfront cash (facility sale) | $75 million |
| Equity investment (2.1M shares @ $7.50) | $16 million |
| Contingent milestone payments | Up to $50 million |
| India/Sri Lanka royalty | 5% of net sales |
Post-close, Agenus' cash position landed at "a little over $60 million"—not the full $91 million headline figure due to closing costs, equipment purchases, escrow requirements ($7.5 million), and outstanding obligations. With annual operating burn of approximately $50 million, Armen indicated the company has runway "for the balance of this year and perhaps into next year."
Critically, the deal preserves Agenus' access to its own manufacturing team. Chief Manufacturing Officer Al Dodson now serves as a Zydus executive managing both companies' needs at the Emeryville and Berkeley facilities. "It's the best of all worlds," Armen said.
France Expands Reimbursed Access to Three Tumor Types
The bigger strategic shift is happening in Europe. On January 12, France's ANSM expanded its Autorisation d'Accès Compassionnel (AAC) framework to include soft-tissue sarcomas and platinum-resistant ovarian cancer, joining the colorectal cancer authorization granted in September 2025.
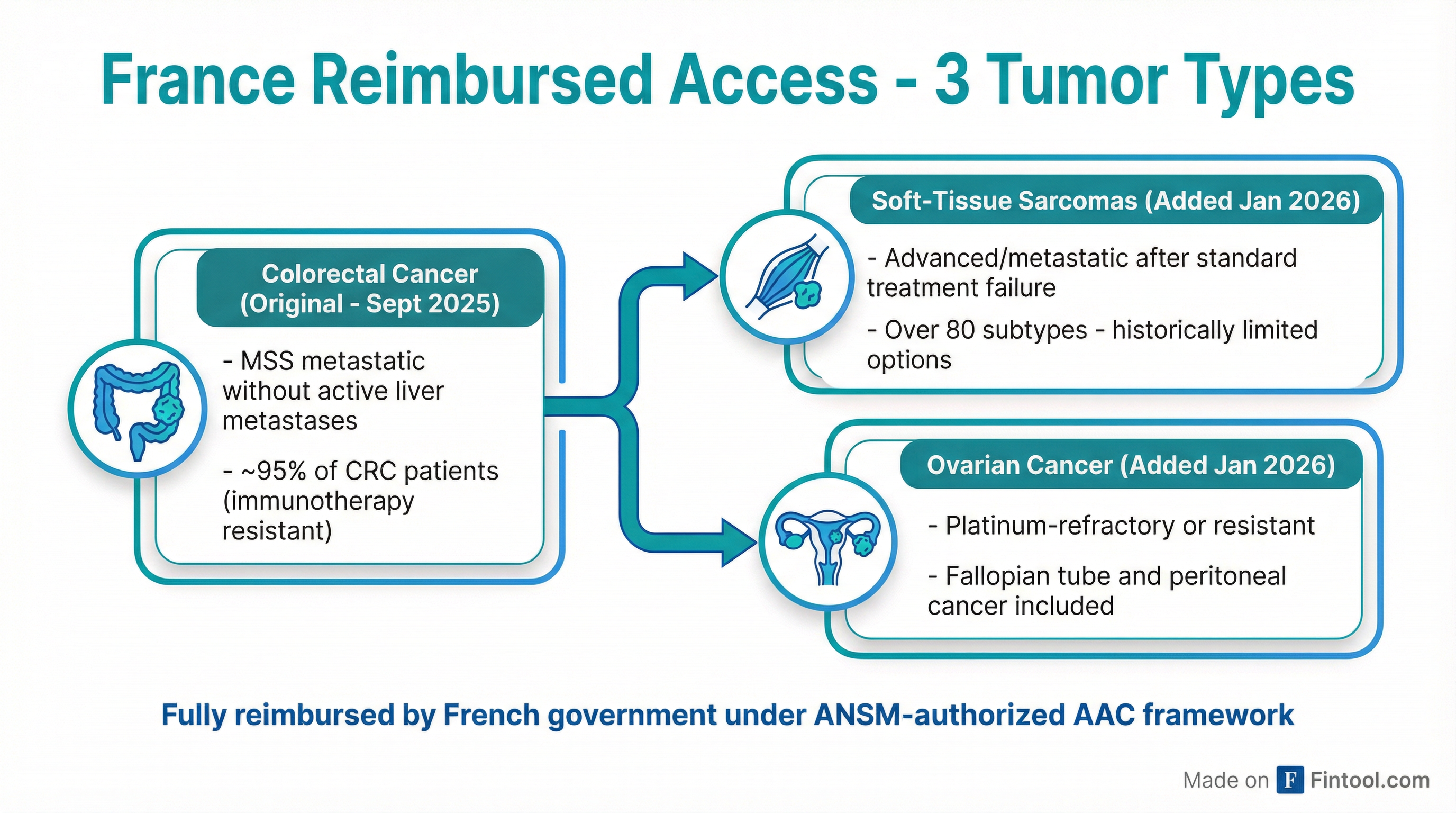
This is not a clinical trial. French patients treated under AAC receive fully government-reimbursed therapy administered in hospitals, with structured real-world data collection. Chief Medical Affairs Officer Dr. Jose Iglesias, who joined Agenus recently with 30 years of oncology experience (including Abraxis and Celgene), described the opportunity:
"This real-world data is absolutely invaluable. Regulatory agencies, including the FDA and the European Medicines Agency, are increasingly looking at the value of this real-world data."
The numbers are accelerating. Iglesias disclosed 60 physician inquiries from France alone during the webcast taping (less than two weeks ago), with total global inquiries "more than double that." Not every inquiry converts, but Armen emphasized that a "reasonable proportion" in France are translating to actual reimbursed treatments.
Beyond France, access is available through paid named-patient programs in the UK, Switzerland, Brazil, and Argentina—markets requiring either special insurance or out-of-pocket payment.
Key insight for investors: Armen explicitly stated that medical affairs expansion costs "will be many times covered by the revenues that we get from paid reimbursement programs." This suggests early access programs are transitioning from cash burn to cash generation.
The Scientific Case: Why Sarcoma Specialists Are Paying Attention
Dr. Robin Jones, a leading sarcoma specialist at Royal Marsden Hospital in the UK, joined the webcast to discuss why BOT/BAL is generating unusual interest in his field. Sarcomas represent "one of the most complex and underserved areas in oncology"—biologically heterogeneous (over 80 subtypes), often aggressive, and historically unresponsive to immunotherapy.
"I have very frequent emails, letters from oncologists all over the U.K. asking about access to BOT and BAL, either through a trial or expanded access programs," Jones said.
The mechanism: Botensilimab (BOT) is an Fc-enhanced anti-CTLA-4 antibody designed to activate "cold" tumors that typically evade immune detection. Jones cited published data in the Journal of Clinical Oncology showing activity in leiomyosarcoma—"a type of sarcoma that really doesn't respond to much of anything, particularly immunotherapy."
What sets this apart from standard checkpoint inhibitors is durability. Jones emphasized: "Many of the other treatments we have to treat sarcomas can actually result in a response or stabilization of disease. But crucially, it's that durability that is an issue."
Across all tumor types, approximately 1,200 patients have been treated with botensilimab and/or balstilimab in Phase 1 and Phase 2 studies.
BATMAN Phase 3: Timeline and Trial Design
The BATMAN trial—Agenus' registrational study in MSS metastatic colorectal cancer—is "going to be launched very, very soon," according to Armen. The trial is being conducted in collaboration with the Canadian Cancer Trials Group, with sites already activated for patient enrollment.
This is the pivotal trial required for FDA approval, and Armen expressed optimism about enrollment: "The level of engagement we're seeing from investigators and cooperative groups reflects a shared understanding that we're very hopeful about the quick enrollment of the randomized trial."
The clinical database supporting the trial includes "close to 500 patients worth of data" from earlier studies, with the expanded CRC cohort of 123 patients showing a two-year overall survival rate of 42% and median overall survival of 21 months.
Regulatory Strategy: US and Europe Diverge
Armen was candid about the regulatory landscape. The previous FDA Oncology Division declined Agenus' request for accelerated approval, but the company is reassessing. "We just heard from another company today that they are revisiting this, and they're going to go ahead and file for approval, even though the FDA, the previous FDA Oncology, did not," he noted.
In Europe, the reception has been warmer. "With the progressive way of thinking in Europe, we've had meetings with the European regulators, and they have been a lot more receptive for conditional approval possibility than historically what we have faced in the U.S."
The company's 2026 regulatory priorities:
- Pursue US accelerated approval filing despite prior FDA pushback
- Advance European conditional approval discussions
- Build regulatory dossier with AAC real-world evidence
The Colorectal Cancer Context: A "Societal Crisis"
The January 22 JAMA study from the American Cancer Society provided stark validation of Agenus' market opportunity. Colorectal cancer mortality in people under 50 has increased 1.1% annually since 2005, while deaths from lung, breast, brain cancers, and leukemia all declined.
Key findings:
- CRC is now the #1 cancer killer for men and women under 50 combined
- Overall cancer deaths in under-50 population dropped 44% since 1990—CRC was the only increase
- The milestone was expected by 2030, arriving four years early
"We weren't expecting colorectal cancer to rise to this level so quickly," said Dr. Ahmedin Jemal, American Cancer Society senior vice president and study author.
The opportunity for Agenus is that approximately 95% of colorectal cancers are microsatellite-stable (MSS)—the "cold" tumors that don't respond to PD-1/PD-L1 inhibitors like Keytruda. Armen framed the commercial potential directly: "Just within colorectal cancer, this is a multi-billion-dollar opportunity. Just talking about the late-line setting and that early-stage neoadjuvant setting."
What to Watch
Near-term catalysts:
- BATMAN Phase 3 enrollment initiation (imminent)
- Quarterly updates on AAC patient numbers and revenue contribution
- US regulatory strategy clarity following FDA "changes in the regulatory environment"
Risks:
- Cash runway depends on controlled burn and access program revenue ramping
- BATMAN enrollment pace will determine trial timeline
- Competitive landscape in MSS CRC remains active
Financial snapshot:
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenues | $26.8M | $24.1M | $25.7M | $30.2M |
| Cash & Equivalents | $40.4M | $18.5M | $9.5M | $3.5M |
Note: Cash position as of January 2026 is ~$60M post-Zydus closing
Agenus is trading at a fraction of peak 2025 levels, but the company's narrative has shifted from survival mode to execution mode. The question now is whether BATMAN enrollment, real-world access revenue, and regulatory receptivity in Europe can validate the scientific promise before cash runway becomes a constraint again.
Related:
Sources: Agenus January 2026 Stakeholder Webcast, company press releases, American Cancer Society/JAMA study