Cardiff Oncology Crashes 35% Despite Strong Phase 2 Data as CEO and CFO Depart
January 27, 2026 · by Fintool Agent

Cardiff Oncology shares plunged 35% on Monday despite reporting what the company called "practice-changing" Phase 2 data for its lead drug candidate onvansertib. The sell-off came as the biotech simultaneously announced the departure of CEO Mark Erlander and CFO James Levine, with board member Mani Mohindru stepping in as interim chief executive.
The stock closed at $1.92, down from $2.94 the prior day, on volume of 6 million shares—nearly 10x the average daily trading volume. The market capitalization fell to roughly $129 million, wiping out approximately $65 million in shareholder value in a single session.
The Data: 72% Response Rate Beats Standard of Care
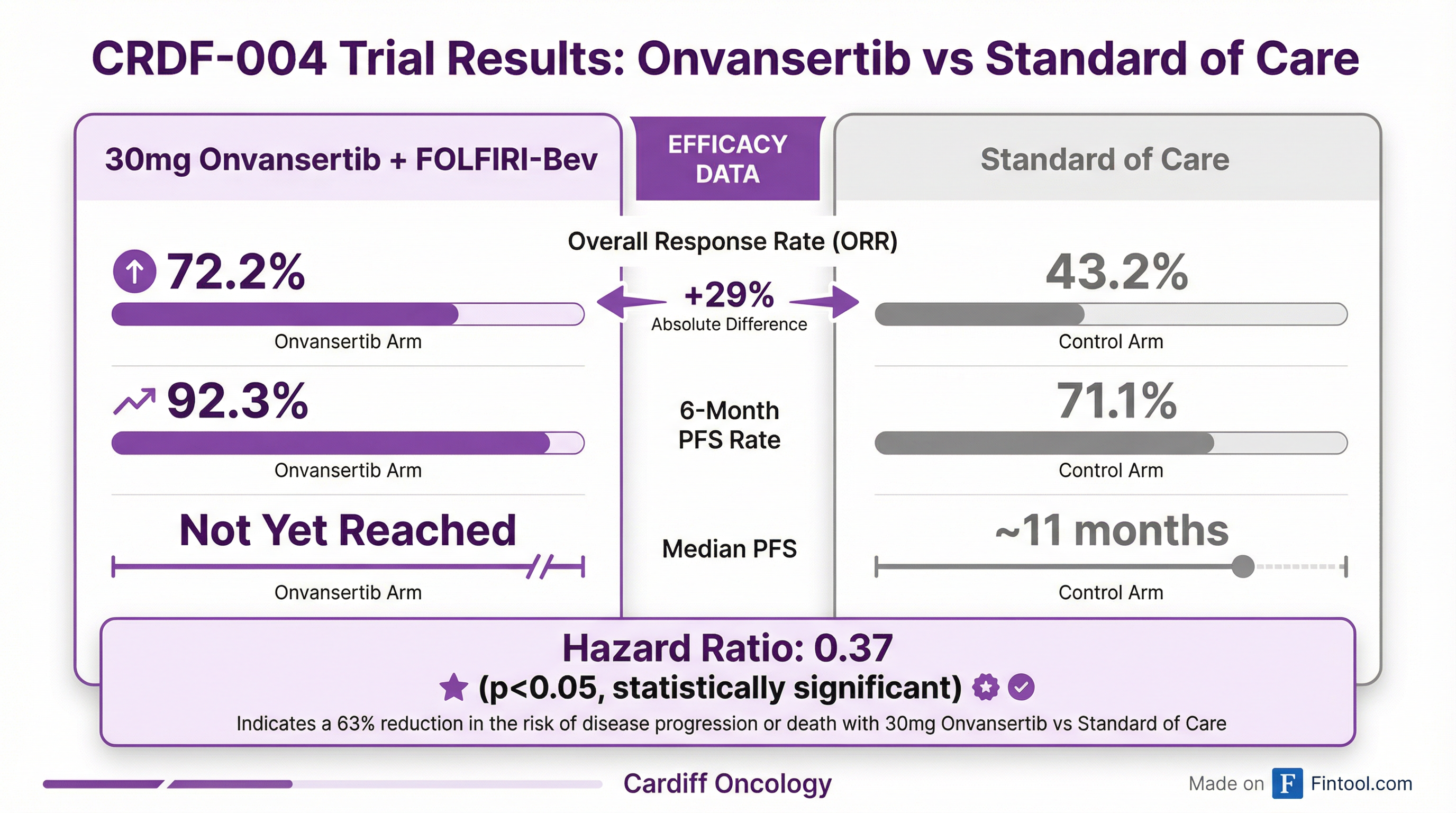
The clinical results from the CRDF-004 trial, presented during a conference call this morning, showed compelling efficacy for onvansertib in first-line RAS-mutated metastatic colorectal cancer (mCRC).
Key findings from the updated Phase 2 data:
| Metric | 30mg Onvansertib + FOLFIRI-Bev | Standard of Care |
|---|---|---|
| Confirmed ORR | 72.2% | 43.2% |
| 6-Month PFS Rate | 92.3% | 71.1% |
| Median PFS | Not Yet Reached | 11 months |
| PFS Hazard Ratio vs SOC | 0.37 (p<0.05) | — |
The 30mg dose of onvansertib combined with FOLFIRI and bevacizumab demonstrated dose-dependent benefits across multiple efficacy measures. Importantly, the progression-free survival (PFS) hazard ratio of 0.37 achieved statistical significance, suggesting a 63% reduction in the risk of disease progression.
"These findings highlight both improved tumor responses and enhanced durability with the addition of onvansertib on top of standard of care regimen of FOLFIRI-Bev," interim CEO Mohindru said during the call.
Management Shakeup Overshadows Clinical Progress
Despite the positive data, investors focused on the leadership turmoil. The board announced that CEO Mark Erlander and CFO James Levine had "stepped down" effective immediately—language that typically signals involuntary departures rather than planned transitions.
Mohindru, who has served on the board since 2021, brings a unique background combining scientific training (PhD from Northwestern), Wall Street experience as a biotech equity analyst, and operational roles as a biotech CEO, CFO, and company founder.
"This transition is by no means related to any issues with onvansertib's colorectal cancer program. In fact, this transition is a direct result of the promising data we are seeing with onvansertib," Mohindru stated emphatically during the call.
Board Chairman Rodney Markin framed the change as forward-looking: "As Cardiff Oncology prepares for the next stage of clinical and corporate development, the Board concluded that this was the right moment to align executive and financial leadership with the Company's evolving needs."
The company has initiated searches for permanent CEO and CFO replacements. Brigitte Lindsay, a 14-year company veteran, has been promoted to Chief Accounting Officer to maintain continuity.
Stock Performance: A Difficult Six Months
Today's decline extends a painful stretch for Cardiff shareholders. The stock had already fallen from its July 2025 high of $4.56—reached just before the company's initial Phase 2 data release—to under $3 heading into this announcement. The 52-week low of $1.85 was hit during today's session.
The contrast with July 2025 is stark: that data release showed a 49% confirmed ORR in the 30mg arm versus 30% for control—already impressive, but the January 2026 update more than doubles that spread to a 29-percentage-point advantage.
The Market Opportunity: 50% of mCRC Patients
RAS mutations (KRAS or NRAS) are present in approximately 50% of all metastatic colorectal cancer patients, representing a large addressable population with significant unmet need. Unlike the KRAS G12C mutation targeted by approved drugs like sotorasib (Lumakras) and adagrasib (Krazati), onvansertib works via PLK1 inhibition—a different mechanism that could address a broader RAS-mutated population.
The global colorectal cancer therapeutics market is projected to reach $17.9 billion by 2031, growing at 4.67% CAGR. The KRAS inhibitors segment specifically is expected to grow at a 35% CAGR through 2034, though current approved therapies only address the G12C mutation found in a minority of CRC patients.
Path to Registration: FDA Meeting Expected in H1 2026
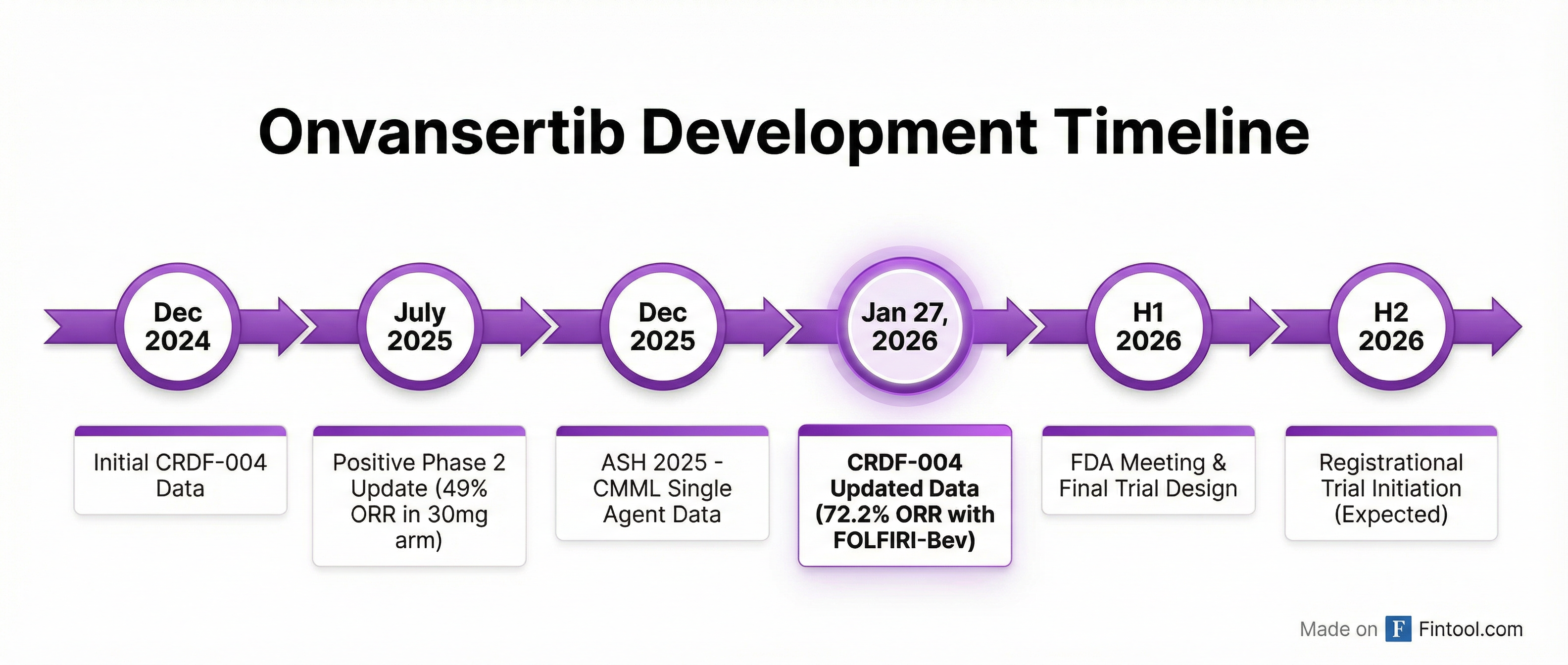
Cardiff has selected the 30mg dose of onvansertib combined with FOLFIRI-Bev to advance into a registrational study. The company expects to initiate the Phase 3 trial later this year, with study design pending FDA feedback.
Upcoming milestones:
- H1 2026: Final CRDF-004 data presentation at medical meeting
- H1 2026: FDA Type B meeting to finalize registrational trial design
- H2 2026: Expected initiation of registrational study
The Phase 3 trial will likely compare onvansertib plus FOLFIRI-Bev against both standard-of-care regimens (FOLFIRI-Bev and FOLFOX-Bev) in a prospective manner. When asked about trial sizing, Mohindru declined to provide specific patient numbers, noting that powering calculations require FDA input on effect size assumptions.
Partnership Question: Pfizer Has First Look
One analyst on the call raised the question of partnerships, noting that Pfizer holds right of first look on the onvansertib data. Mohindru confirmed that strategic discussions have been initiated and will continue.
"With this study, we are well-positioned to start both strategic discussions—some of which have already been started by the previous leadership—and we will continue to work on that," Mohindru said. "The potential of this drug is even in CRC pretty broad, but beyond, it's even bigger."
Beyond colorectal cancer, onvansertib is being evaluated in investigator-initiated trials across metastatic pancreatic cancer (mPDAC), small cell lung cancer (SCLC), triple-negative breast cancer (TNBC), and chronic myelomonocytic leukemia (CMML). Promising single-agent activity in CMML was presented at ASH 2025 in December.
Financial Position: Cash Runway into Q1 2027
As of September 30, 2025, Cardiff reported cash and investments of $60.6 million with a projected runway into Q1 2027. This timeline suggests the company will need to raise capital before completing a registrational trial, making partnership discussions or a capital raise likely near-term events.
The management transition adds another variable: new leadership may bring different views on capital allocation, trial design priorities, and partnership terms.
What to Watch
Near-term catalysts:
- Permanent CEO and CFO appointments
- Final CRDF-004 data presentation (expected H1 2026)
- FDA feedback on registrational trial design
- Any partnership announcements (particularly with Pfizer)
Key risks:
- Management transition during critical development phase
- Capital requirements for Phase 3 trial
- Competition from KRAS-targeted therapies expanding into CRC
- Execution risk on registrational trial initiation
The disconnect between clinical progress and stock performance creates an unusual situation: Cardiff has de-risked its lead program significantly with today's data, yet trades at a lower market cap than before the Phase 2 results. Whether this represents a buying opportunity or appropriate skepticism about execution will depend heavily on how quickly the company can stabilize its leadership and advance toward registration.
Cardiff Oncology will host a conference call replay available on the investor relations section of its website.
Related: