Fractyl's Revita Shows 70% Weight Regain Reduction in Key Subgroup—But Shares Sink 14%
January 29, 2026 · by Fintool Agent
Fractyl Health shares tumbled 14% Wednesday after the company released six-month data from its REMAIN-1 midpoint cohort that showed meaningful weight maintenance in patients who discontinued GLP-1 drugs—but missed statistical significance in the primary analysis.
The medical device company's Revita procedure demonstrated a 70% reduction in weight regain among patients who had lost above-median weight on tirzepatide, with a highly significant p-value of 0.004. But the full efficacy population told a less convincing story: Revita patients regained 4.5% of body weight versus 7.5% for sham—a p-value of just 0.07.
The stock closed at $1.83, down from $2.12, with aftermarket trading showing prices as low as $0.67—suggesting further selling pressure as investors digest the mixed results.
The Data: Strong Signal, Weak Statistics
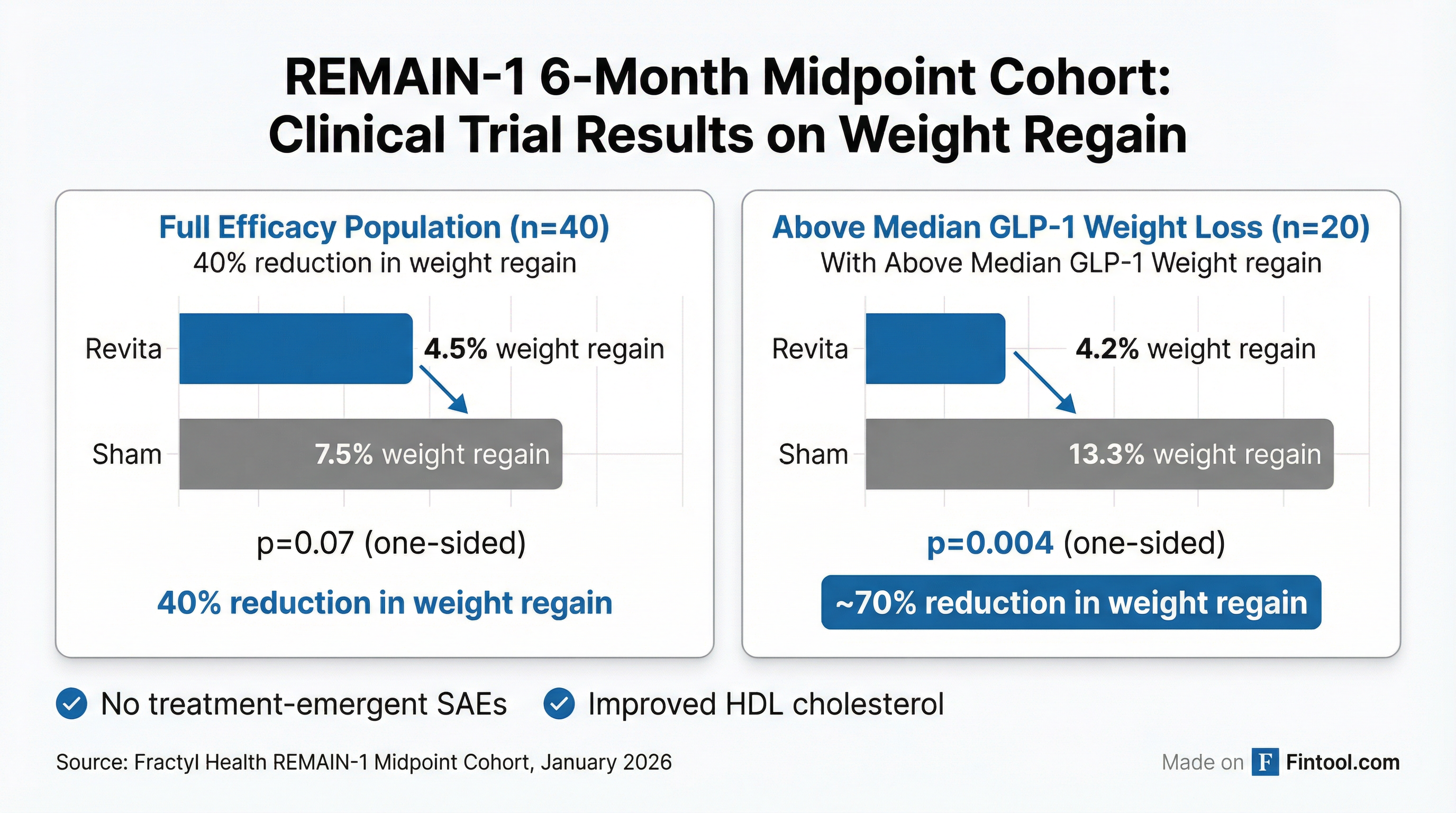
The REMAIN-1 midpoint cohort enrolled 45 adults with obesity who achieved at least 15% total body weight loss on tirzepatide before being randomized 2:1 to Revita versus sham. After accounting for diet and lifestyle noncompliance, the efficacy population included 40 patients—29 in the Revita arm and 11 in sham.
| Metric | Revita | Sham | Difference | P-value |
|---|---|---|---|---|
| Weight regain (full population, n=40) | 4.5% | 7.5% | -3.0 pp | 0.07 |
| Weight regain (above median weight loss, n=20) | 4.2% | 13.3% | -9.1 pp | 0.004 |
| HDL cholesterol improvement | +15.5 mg/dL | +3.9 mg/dL | +11.6 | 0.01 |
| Sweet food craving reduction | 1.8 | 3.4 | -1.6 | 0.04 |
CEO Harith Rajagopalan acknowledged one clinical site contributed to higher-than-expected weight regain in both arms. "This site is not contributing materially to the pivotal cohort," he said on the investor call. When backing out that site, Revita's average weight regain drops to less than 2.5%—in line with the company's open-label REVEAL-1 data showing just 1.5% regain at six months.
Why the Market Isn't Buying It
The disconnect between Fractyl's "compelling" framing and the market's reaction comes down to one number: 0.07.
In biotech, a p-value of 0.05 or lower is the traditional threshold for statistical significance. At 0.07 in a small pilot study with 40 patients, the signal is suggestive but not conclusive. While management argues this "bodes very well for the pivotal study design with a sample size of 315 patients," investors are pricing in execution risk.
The subgroup analysis showing 70% reduction in weight regain is genuinely striking—but exploratory subgroup analyses, by definition, aren't the pre-specified primary endpoint. Regulatory approval depends on the pivotal trial hitting its six-month primary endpoint later this year.
The Bigger Picture: GLP-1 Weight Regain Is a $100B+ Problem
Fractyl is betting on a therapeutic category that barely existed two years ago: post-GLP-1 weight maintenance.
A systematic review published in The BMJ this month found that patients stopping GLP-1 drugs like Wegovy and Zepbound regain weight at 0.8 kg per month, returning to baseline within 18 months. That's an average of 10 kg (22 pounds) regained in the first year alone—wiping out most of the benefit from drugs that cost $1,000+ per month.
With an estimated 50% of GLP-1 users discontinuing within 12 months due to cost, insurance issues, or side effects, Fractyl sees a massive addressable market.
"We believe weight loss maintenance is the new unmet need in obesity care," Rajagopalan said in a January outlook statement. "With millions of patients expected to start and then stop taking a GLP-1 this year, this is clearly what patients, prescribers, payers, and the broader health economic community are looking for."
Regulatory Path: De Novo Could Be a Game-Changer
In a potentially significant development, Fractyl disclosed it has requested FDA feedback on reclassifying Revita under the De Novo pathway instead of the more burdensome Premarket Approval (PMA) route.
| Pathway | Risk Class | FDA Timeline | Clinical Evidence | Post-Approval |
|---|---|---|---|---|
| De Novo | Class I/II (low-moderate) | 150 FDA days | Scaled to risk | Creates predicate for 510(k)s |
| PMA | Class III (high) | 180 FDA days | Extensive clinical data | No predicate created |
FDA feedback on the De Novo request is expected in Q2 2026. If granted, it could mean a faster, more capital-efficient path to market.
2026 Catalyst Calendar
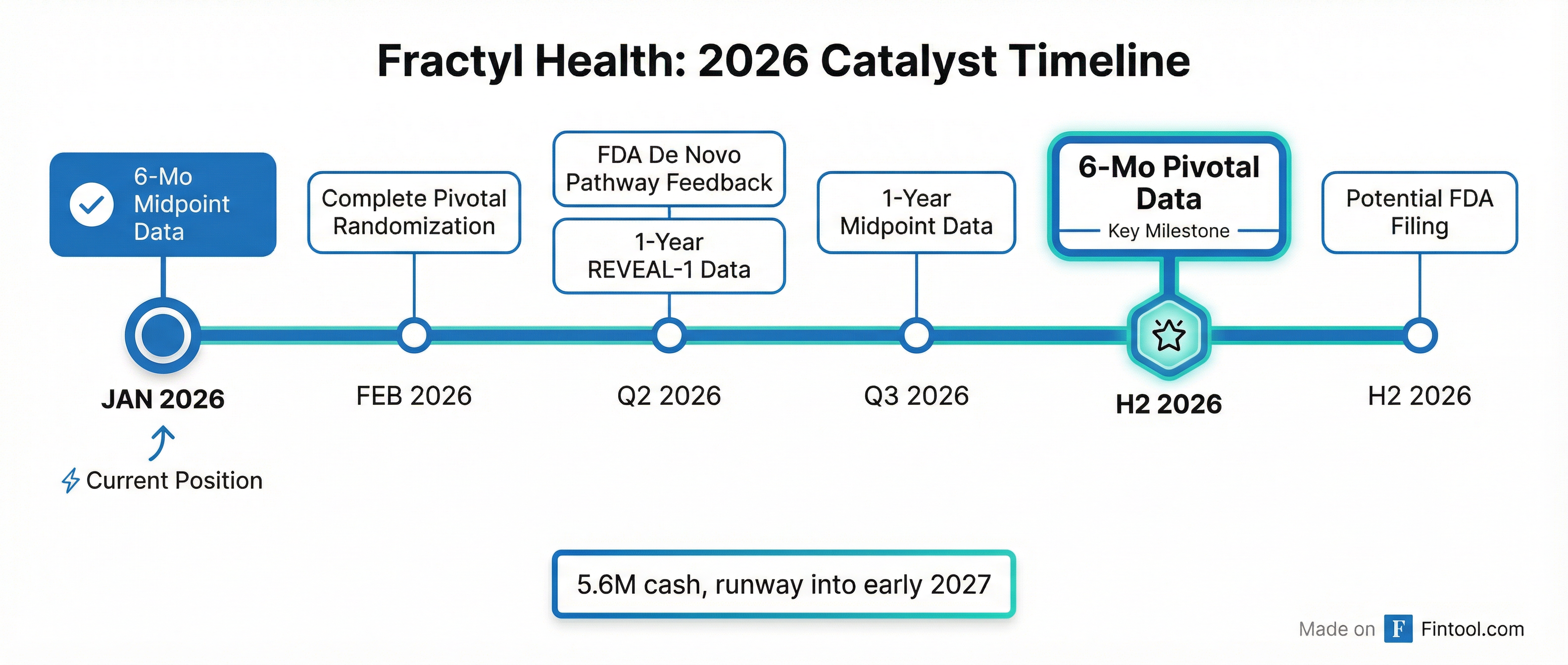
Fractyl has a busy year ahead, with the pivotal REMAIN-1 cohort now 95% randomized:
- February 2026: Complete pivotal cohort randomization
- Q2 2026: FDA feedback on De Novo pathway
- Q2 2026: 1-year REVEAL-1 open-label data
- Q3 2026: 1-year REMAIN-1 midpoint cohort data
- H2 2026: Pivotal 6-month primary endpoint data
- H2 2026: Potential FDA filing
Financial Position: Runway to Early 2027
Fractyl ended Q3 2025 with $77.7 million in cash, and reported approximately $85.6 million as of early January 2026 after receiving $23 million in warrant exercise proceeds. Management says this provides runway into early 2027.
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Cash & Equivalents | $67.5M | $42.1M | $22.3M | $77.7M* |
| Net Loss | ($25.0M) | ($23.7M) | ($27.9M) | ($45.6M)* |
*Values retrieved from S&P Global
The Q3 cash increase reflects the August 2025 financing. Burn rate has accelerated as the company ramps pivotal trial execution.
The Bottom Line
Fractyl's REMAIN-1 midpoint data tells two stories. For bulls, a 70% reduction in weight regain in high-responders with p=0.004 validates the biological mechanism and suggests the pivotal trial—with 8x more patients—should succeed. The De Novo pathway request, if granted, could meaningfully accelerate the path to market.
For skeptics, a p=0.07 in a 40-patient pilot, combined with acknowledged site quality issues and the need for post-hoc subgroup enrichment to find significance, raises questions about whether the pivotal will deliver.
The next read-through comes in H2 2026, when 315-patient pivotal data will determine whether Revita becomes the first durable procedural therapy for post-GLP-1 weight maintenance—or another promising idea that couldn't clear the FDA bar.
Related: Fractyl Health Company Profile