IO Biotech's CMO Exits as Cancer Vaccine Developer Engages Bankers for Potential Sale
January 30, 2026 · by Fintool Agent
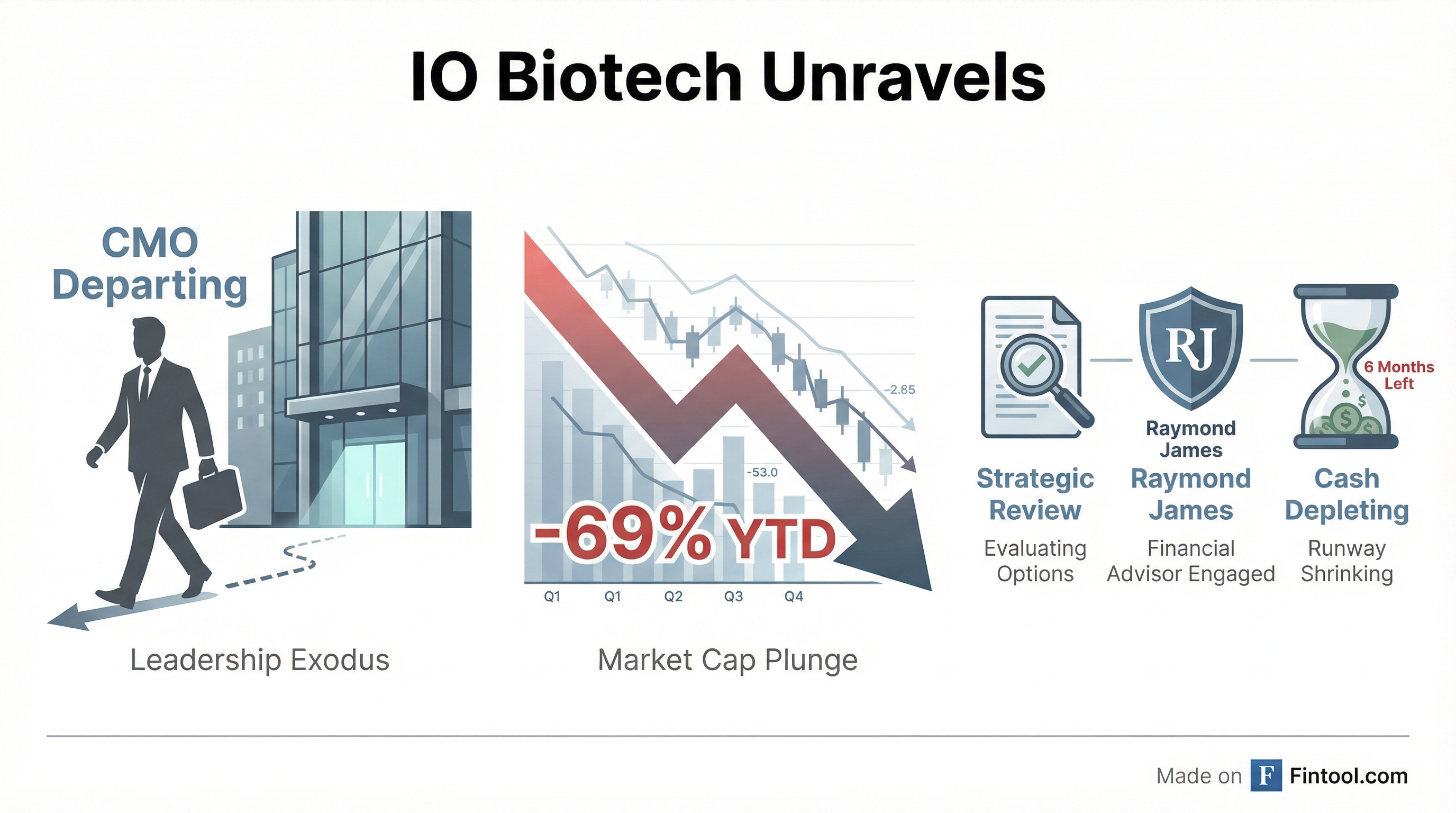
Io Biotech+9.27%'s Chief Medical Officer Dr. Qasim Ahmad is departing the company effective February 15, 2026, the latest casualty in a five-month unraveling that has seen the Danish cancer vaccine developer lose its lead drug candidate, slash its workforce twice, and hire investment bankers to explore a sale or liquidation.
The stock fell 9% today to $0.29 on heavy volume, extending a 69% decline since the start of 2025. Market capitalization has collapsed from roughly $140 million before the FDA setback in September to approximately $15 million today.
Raymond James Engaged as Financial Advisor
IO Biotech disclosed Friday that it has retained Raymond James & Associates as exclusive financial advisor to assist in its previously announced exploration of strategic alternatives. Options on the table include a merger, business combination, asset sale, or—notably—liquidation and dissolution.
The company is simultaneously implementing "cost-containment and cash conservation measures, including a significant reduction of the company's workforce." This marks the second round of layoffs in four months. In September 2025, IO Biotech cut approximately 50% of its workforce after the FDA rejected its path to approval.
The latest restructuring is expected to cost $2.4–$2.6 million in severance and related charges, primarily in Q1 2026.
The CMO Who Oversaw the Trial That Failed
Dr. Ahmad, a trained clinical oncologist with over two decades of experience, joined IO Biotech in July 2023 from Novartis, where he served as Senior Vice President and US Head of Clinical Development & Medical Affairs for the oncology business unit.
He was hired specifically to shepherd IO Biotech's lead candidate Cylembio through FDA approval—a mission that ultimately failed. The Phase 3 IOB-013 trial enrolled 407 patients at over 100 sites worldwide, testing the cancer vaccine in combination with Merck's Keytruda for first-line advanced melanoma.
In transcript after transcript, Dr. Ahmad championed the data. In September 2025, just weeks before the FDA delivered its verdict, he told Morgan Stanley analysts: "When we look at the data, data is quite convincing... the risk-benefit in this needs to be kept into consideration."
It wasn't enough.
Five Months of Collapse

The unraveling began on August 11, 2025, when IO Biotech reported topline Phase 3 results. Cylembio plus pembrolizumab demonstrated clinical improvement over pembrolizumab alone—19.4 months median progression-free survival versus 11.0 months—but the p-value of 0.056 narrowly missed the pre-specified threshold of 0.045.
The stock cratered 44% that day, falling from $1.88 to $1.05.
Management initially sounded optimistic. CEO Mai-Britt Zocca told investors the company would approach the FDA with "the totality of the evidence," citing precedent for approvals despite marginal p-value misses.
That optimism was crushed on September 29, 2025. After a pre-BLA meeting, the FDA recommended that IO Biotech not submit a Biologics License Application based on the IOB-013 data. The stock collapsed to $0.37 that day from $1.58 two days earlier—a 77% two-day wipeout.
The same day, the company announced it would cut 50% of its workforce and admitted its cash would only fund operations through Q1 2026.
By January 21, 2026, with no FDA path forward and cash dwindling, the Board pulled the trigger on a full strategic review. The stock crashed another 56% to $0.21 on the announcement.
Cash Runway Running Out
IO Biotech's latest 10-Q paints a dire picture. The company had $30.7 million in cash as of September 30, 2025, which it stated "will not be sufficient to fund our operating expenses and capital requirements for at least 12 months."
The filing included a going concern warning: "Our recurring losses from operations raise substantial doubt about our ability to continue as a going concern."
Since inception, the company has accumulated losses of $416.3 million.
| Metric | Value |
|---|---|
| Cash & Equivalents (Sep 30, 2025) | $30.7M |
| Accumulated Deficit | $416.3M |
| Q1-Q3 2025 Net Loss | $57.0M |
| Cash Burn (9 mo) | $61.4M |
| Runway | Through Q1 2026 |
What Remains
IO Biotech still holds some assets that could attract buyers:
- T-win Platform: The company's proprietary cancer vaccine technology designed to activate T cells against both tumor cells and immune-suppressive cells in the tumor microenvironment
- Clinical Data: Five years of Phase 1/2 and Phase 3 data in melanoma, plus Phase 2 data in head and neck cancer and NSCLC
- European Path: Management has discussed seeking approval in Europe based on the IOB-013 data, though success is uncertain
- Second Asset (IO112): A preclinical cancer vaccine targeting arginase 1, with IND submission planned for 2025
But without a CMO to lead clinical development, without the cash to run a new Phase 3 trial, and with a strategic review process explicitly including liquidation as an option, the endgame appears near.
The Signal in the CMO Departure
At clinical-stage biotechs, the Chief Medical Officer is often the last executive to leave before the lights go out. They're essential to any deal that involves continuing clinical development—acquirers want the scientific continuity.
Dr. Ahmad's departure suggests one of two things: either IO Biotech expects to sell its assets rather than the company as an ongoing concern, or potential acquirers have indicated they'll bring their own clinical leadership.
Either way, the departure removes a key piece of the institutional knowledge that made Cylembio possible. Dr. Ahmad led the pre-BLA strategy, presented to the FDA, and testified to the drug's risk-benefit profile. His exit, effective in two weeks, leaves CEO Mai-Britt Zocca and CFO Amy Sullivan to shepherd the strategic process to its conclusion.
What to Watch
- Strategic Process Timeline: IO Biotech said it has established no timetable for completing the review and "does not expect to disclose developments unless and until the Board has concluded that disclosure is appropriate."
- Cash Burn: With the runway ending in Q1 2026, the company has weeks to months before difficult decisions become unavoidable
- European Regulatory Path: Whether IO Biotech pursues a Marketing Authorization Application in Europe could signal the value it places on Cylembio's remaining potential
Related Companies: Io Biotech+9.27%