Korro Bio Unveils $3.5B RNA Editing Play at Analyst Day, Eyes 2026 Clinical Entry
January 27, 2026 · by Fintool Agent

Korro Bio laid out its vision for KRRO-121, a first-in-class RNA editing therapy targeting a combined $3.5 billion market opportunity in ammonia-driven diseases, at its virtual Analyst Day today. The presentation marks a strategic pivot for the Cambridge-based biotech following mixed results from its lead program, as the company doubles down on a potentially transformative approach to treating urea cycle disorders (UCD) and hepatic encephalopathy (HE).
Shares of KRRO traded at $10.46, down 2.5% on the day, as investors digested the company's clinical development timeline and market positioning. The stock has recovered significantly from its October lows near $5 following the KRRO-110 setback, but remains well below its 52-week high of $55.89.
The Billion-Dollar Ammonia Problem
Today's presentation, featuring hepatologist Dr. Bruce Scharschmidt (former CMO of Hyperion Therapeutics) and UCD parent Michelle Dinon, hammered home the substantial unmet need in ammonia control.
"Urea Cycle Disorders are cruel and unforgiving," Dr. Scharschmidt emphasized, noting that patients face severely protein-restricted diets, medications requiring 3-4x daily dosing, and the constant threat of hyperammonemic crises that can cause permanent disability or death.
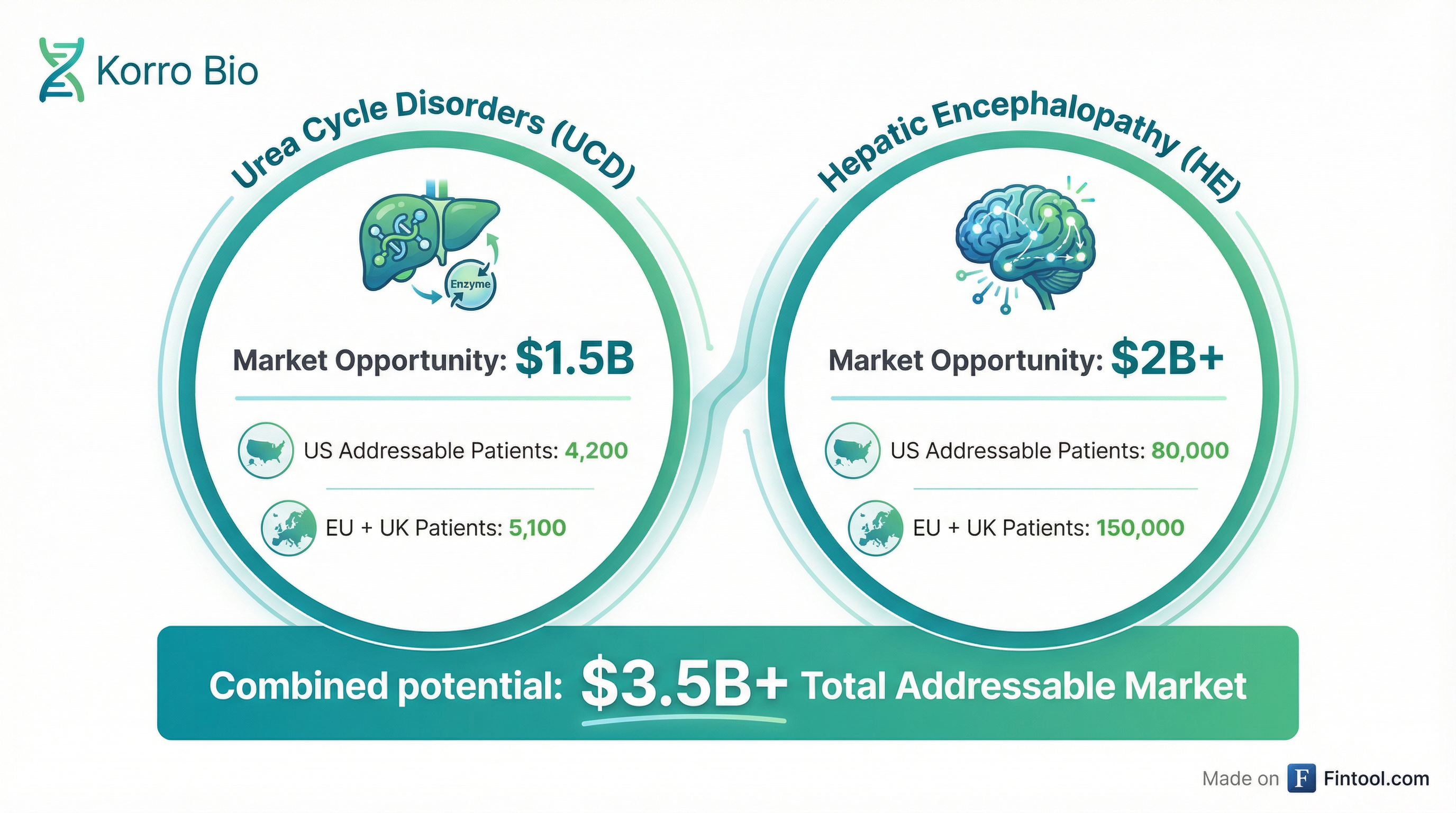
Korro's management outlined addressable patient populations and market potential that dwarfs the company's current ~$100 million market capitalization:
| Indication | US Patients | EU + UK Patients | Market Opportunity |
|---|---|---|---|
| Urea Cycle Disorders | 4,200 | 5,100 | $1.5B |
| Hepatic Encephalopathy | 80,000 | 150,000 | $2B+ |
| Combined | 84,200 | 155,100 | $3.5B+ |
Why Current Treatments Fall Short
Dr. Scharschmidt's clinical experience at Hyperion Therapeutics—which developed Ravicti (glycerol phenylbutyrate)—provided unique insight into the limitations of existing nitrogen-scavenger therapies.
Current UCD treatment challenges:
- Ravicti costs approximately $793,000 per year per patient
- Requires multiple daily doses and severely restricted diets
- Sodium phenylbutyrate causes unpleasant body odor
- Both drugs have a narrow therapeutic index with potential for phenylacetic acid toxicity
- Non-compliance is a major driver of hyperammonemic crises
For hepatic encephalopathy, the picture is equally challenging. Despite rifaximin (Xifaxan) commanding 25-30% market share in the HE treatment market valued at ~$1.7 billion, 82% of patients experiencing HE events in observational studies were already on rifaximin.
"HE is still a big and expensive problem," noted Dr. Scharschmidt, citing HE hospitalization costs exceeding $10,000 per event and total US costs in the billions.
KRRO-121: A New Mechanism for Ammonia Clearance
Korro Bio's Chief Scientific Officer, Dr. Loïc Vincent, detailed the novel approach underlying KRRO-121—one that fundamentally differs from existing nitrogen-scavenger therapies.
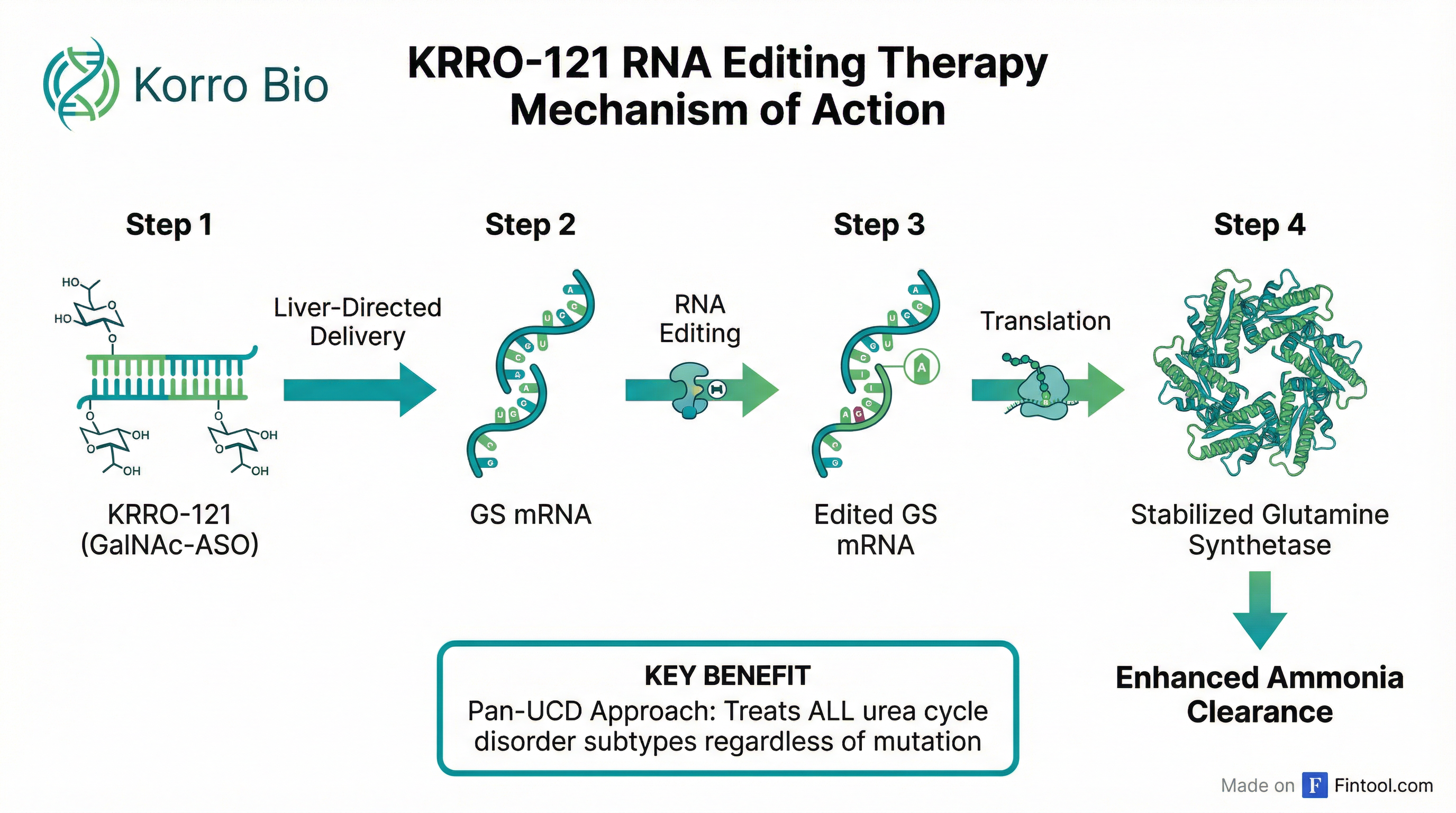
Key mechanism highlights:
-
Stabilized Glutamine Synthetase (GS): KRRO-121 is designed to create a de novo GS protein variant that resists degradation, providing sustained ammonia clearance capacity
-
Liver-Targeted Delivery: GalNAc conjugation ensures liver-specific RNA editing, avoiding systemic effects
-
Pan-UCD Approach: Unlike gene therapies targeting specific mutations, KRRO-121 can potentially treat ALL UCD subtypes regardless of mutational background
-
Convenient Administration: Subcutaneous delivery designed for chronic maintenance therapy
The company presented preclinical data showing KRRO-121 stabilized GS in OTC-deficient iPSC-derived hepatocytes, supporting the therapeutic hypothesis.
Target Product Profile and Differentiation
COO Todd Chappell outlined KRRO-121's target product profile, emphasizing the potential for differentiation across multiple dimensions:
For UCD patients:
- Pan-UCD approach treating all subtypes
- Convenient subcutaneous delivery
- Potential reduction in hyperammonemic crises
- Diet liberalization opportunity
For HE patients:
- Direct ammonia control mechanism
- Convenient subcutaneous delivery
- Potential reduction in HE events
- Improved survival and quality of life
The company highlighted that HE patients with elevated ammonia (≥1.5x ULN) and stable liver function experience more than 2-fold higher HE-related hospitalization rates, underscoring the pharmacoeconomic opportunity.
Stock Performance and Investor Considerations
| Metric | Value |
|---|---|
| Current Price | $10.46 |
| Change (Day) | -2.5% |
| 52-Week Range | $5.20 - $55.89 |
| Market Cap | $98.5M |
| 50-Day Average | $8.20 |
| Cash Position (Q3 2025) | $102.5M |
The stock's dramatic decline from its 52-week high reflects the November 2025 KRRO-110 disappointment, when the company's alpha-1 antitrypsin deficiency (AATD) program achieved proof-of-concept but fell short of therapeutic protein thresholds. That setback prompted a 34% workforce reduction and strategic pivot to GalNAc-conjugated constructs.
However, the recent recovery from October lows suggests investors see potential in the KRRO-121 program. The company's cash runway extends into 2H 2027, providing sufficient capital to advance KRRO-121 into the clinic and potentially deliver initial human data.
What to Watch
Near-term catalysts:
- Regulatory filing for KRRO-121 first-in-human trial expected 2H 2026
- GalNAc-conjugated AATD development candidate nomination expected 1H 2026
Key risks:
- Pre-clinical to clinical translation risk—KRRO-110 results showed differences between preclinical projections and human outcomes
- Competition from established treatments and other gene therapy approaches
- Cash runway extends only to 2H 2027, potentially requiring additional financing
Competitive context: The Ravicti market represents approximately $398 million in 2024 sales and is projected to reach $627 million by 2031. The broader UCD treatment market is growing at 3-7% CAGR, while the HE treatment market is expected to reach $3 billion by 2035.
The Bottom Line
Korro Bio's Analyst Day presentation delivered a compelling narrative around the unmet need in ammonia-driven diseases and KRRO-121's potential as a first-in-class treatment. The combined $3.5B+ market opportunity against a ~$100M market cap creates significant upside if the company can successfully translate its preclinical observations into human proof-of-concept.
However, the path is not without risk. The KRRO-110 experience serves as a reminder that preclinical success doesn't always translate to clinical outcomes. With regulatory filing expected in 2H 2026 and limited cash runway, Korro will need to demonstrate compelling early clinical data to access capital markets on favorable terms.
For investors willing to accept binary clinical-stage biotech risk, KRRO offers exposure to an emerging modality (RNA editing) in validated therapeutic areas with substantial commercial potential. The next 18 months will be critical in determining whether Korro Bio's vision can become reality.
Related:
Photo: Korro Bio