Korro Bio Unveils KRRO-121 Preclinical Data at Analyst Day, Targets $3.5B Ammonia Control Market
January 27, 2026 · by Fintool Agent
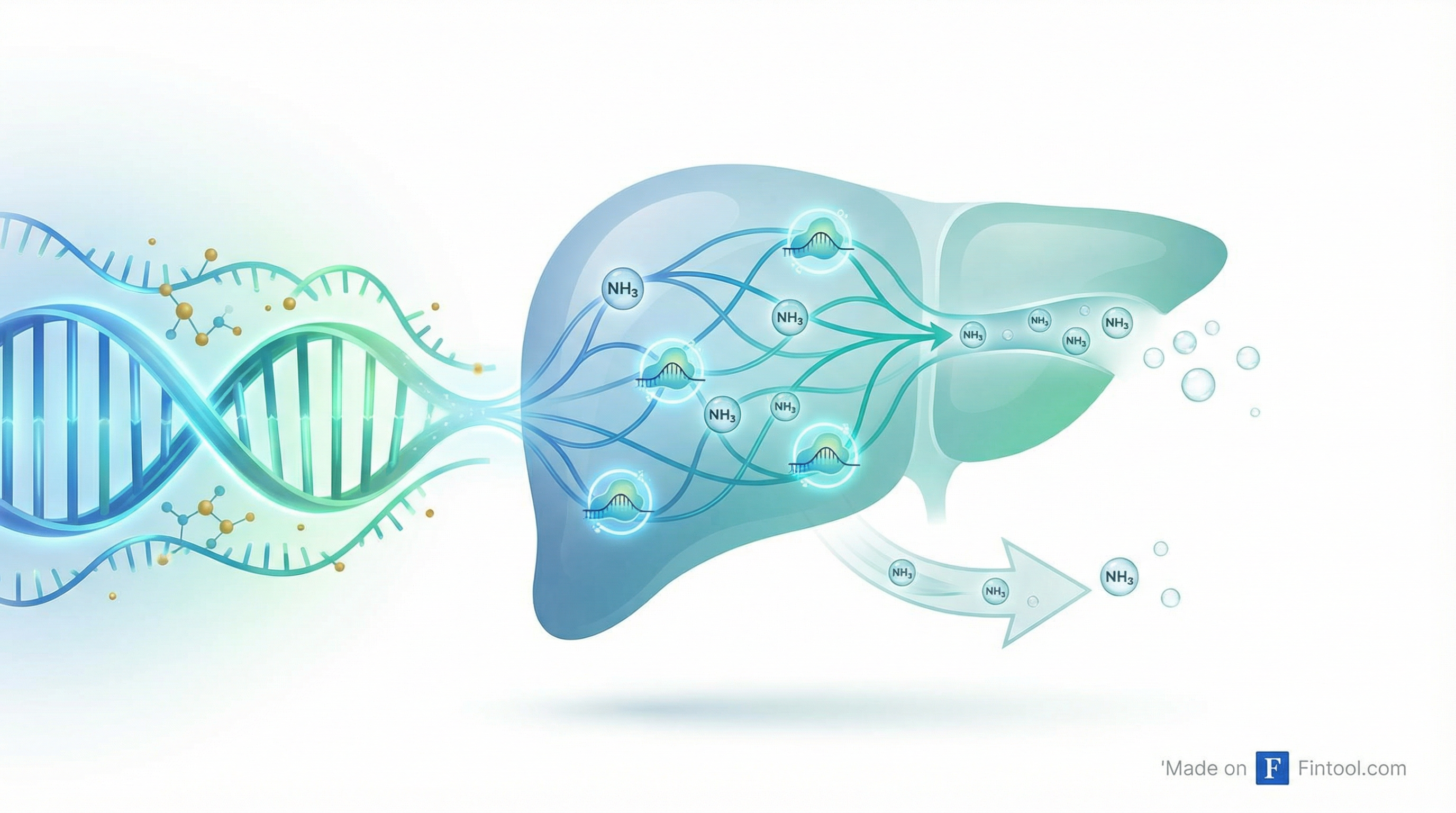
Korro Bio is making its second act pitch to investors. Less than three months after its lead RNA editing program crashed and burned—sending shares down 80% in a single day—the Cambridge biotech is back with detailed preclinical data on a new candidate it says could transform ammonia control in patients with urea cycle disorders and hepatic encephalopathy.
At its virtual Analyst Day this morning, Korro unveiled KRRO-121, a GalNAc-conjugated oligonucleotide that takes an entirely different approach to genetic medicine: rather than correcting a mutation, it creates a brand-new protein variant with enhanced therapeutic function. The company is targeting regulatory filing for first-in-human studies in the second half of 2026, with a combined addressable market exceeding $3.5 billion.
Shares traded down 2.6% to $10.45 midday, hovering near their post-crash levels—a far cry from the $55 highs seen in October before the KRRO-110 disappointment.
The Science: Bypassing the Urea Cycle
The elegance of KRRO-121 lies in its mechanism. Rather than trying to fix the broken urea cycle enzymes that cause inherited metabolic disorders, Korro is activating an alternative pathway—glutamine synthetase (GS)—that can clear ammonia independently.
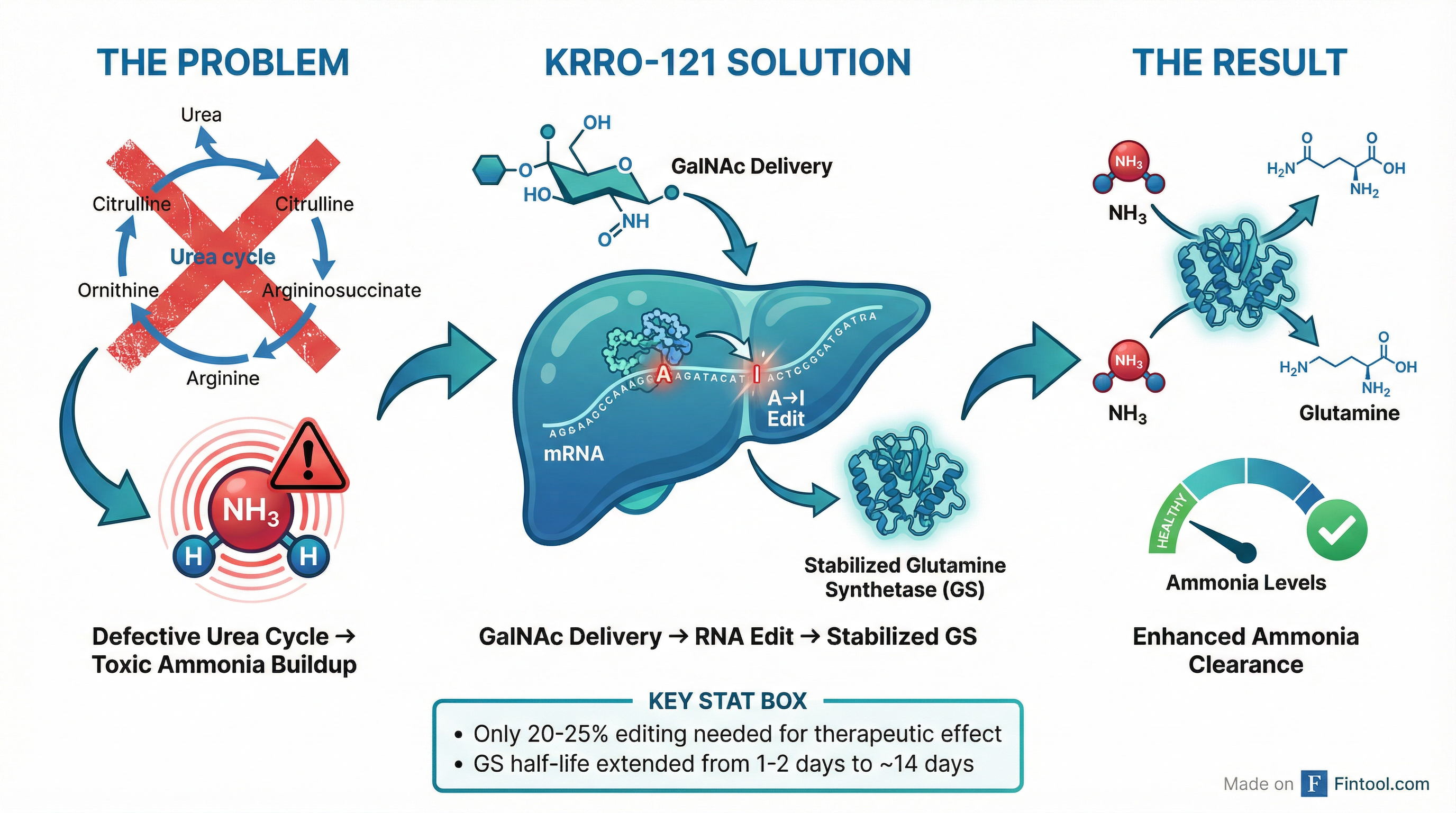
"We're not correcting a mutation. You don't need 90% editing," explained Chief Scientific Officer Loïc Vincent during the presentation. "With much lower editing, you can really correct a pathway, and you can restore a normal function."
The genetic validation is compelling. Korro identified nine patients with naturally occurring start-loss variants that stabilize GS—and these individuals show increased GS stability, maintained enzyme activity, and critically, lower ammonia levels. KRRO-121 is designed to recreate this protective biology using RNA editing.
Key preclinical findings presented include:
| Finding | Data |
|---|---|
| Editing efficiency needed | 20-25% for full therapeutic effect |
| GS half-life extension | From 1-2 days to 14 days |
| Ammonia reduction (OTC-deficient mice) | Dramatic reduction to near-normal limits |
| Humanized mouse model (PXB) | 20-30% reduction in basal ammonia |
| CNS exposure | No brain distribution, no astrocyte effects |
| Liver targeting | >90% delivery to hepatocytes |
A Clinical Expert's Endorsement
The presentation featured Bruce Scharschmidt, MD, the former Chief Medical Officer of Hyperion Therapeutics who developed Ravicti—the current standard of care for urea cycle disorders. Hyperion was acquired by Horizon Pharma for $1.1 billion in 2015.
Scharschmidt's participation sends a clear signal: KRRO-121 isn't just another preclinical compound—it's being developed with deep expertise in the disease area.
"The advantages of 121 should be substantial," Scharschmidt said. "Compared with very short half-life PAA prodrugs, durable around-the-clock coverage should benefit all patients, even those with good compliance, for example, where they sleep or if they're too sick to take their meds."
The current treatments—Ravicti and Buphenyl—require dosing up to three times daily and still fail to prevent hyperammonemic crises that can cause brain damage or death. Ravicti carries an annual price tag of approximately $794,000 per patient.
The November 2025 Collapse—And What Changed
Understanding today's presentation requires context. In November, Korro reported that KRRO-110—its lead candidate for alpha-1 antitrypsin deficiency—produced functional protein but at levels far below what was projected from preclinical data. The result was catastrophic: an 80% stock drop, 34% workforce reduction, CMO resignation, and Novo Nordisk pausing a partnership worth up to $530 million.
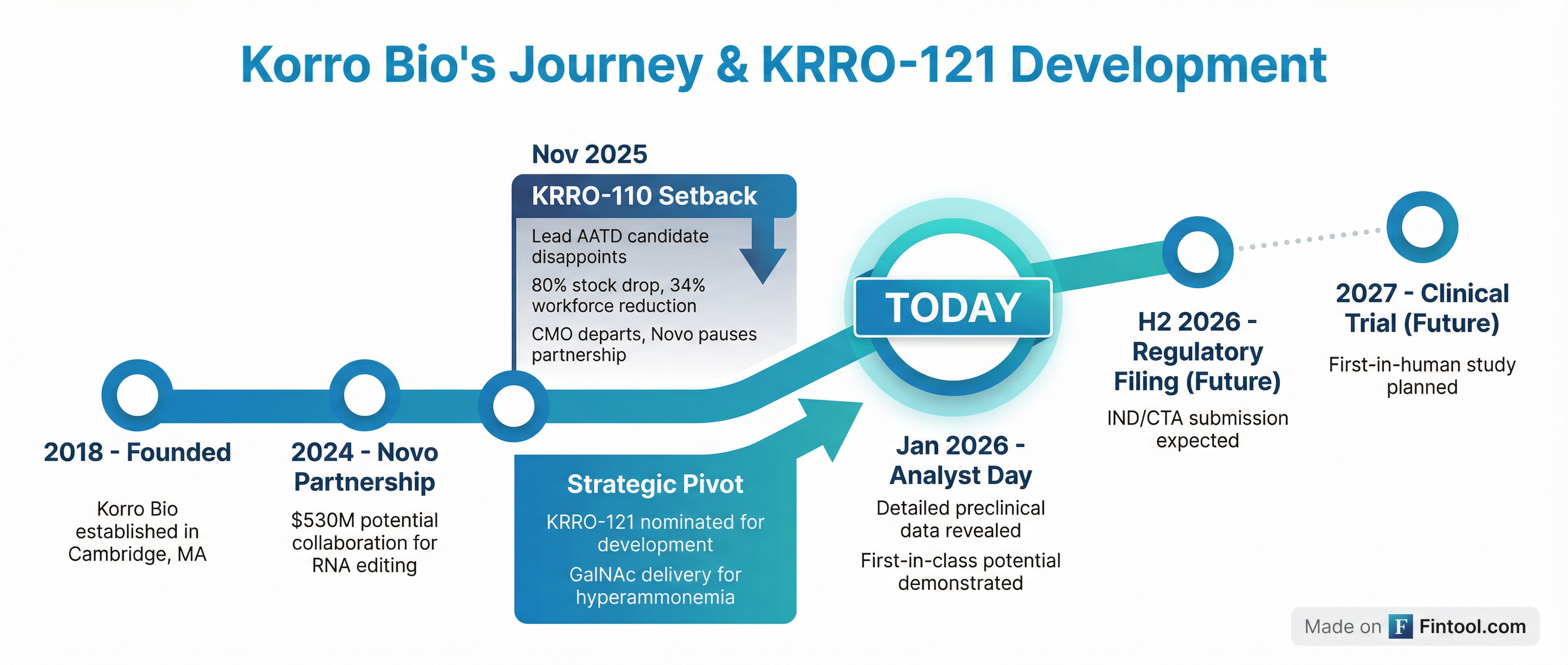
But KRRO-121 is fundamentally different:
1. Different delivery mechanism: KRRO-110 used lipid nanoparticles (LNPs); KRRO-121 uses GalNAc conjugation, which provides "exquisite liver selectivity" with >90% delivery to hepatocytes.
2. Different therapeutic approach: KRRO-110 attempted to correct a missense mutation requiring high editing efficiency. KRRO-121 creates a de novo stabilized protein variant, requiring only 20-25% editing for therapeutic effect.
3. Improved potency: CEO Ram Aiyar noted that KRRO-121 is "couple of logs more potent" than the GalNAc constructs Korro had previously developed.
The Market Opportunity
Todd Chappell, Korro's COO, laid out a substantial addressable market spanning two disease areas:

Urea Cycle Disorders
| Metric | Value |
|---|---|
| US genetic frequency-implied patients | 66,500 |
| Severe post-neonatal onset (addressable) | 4,200 (US) + 5,000 (Europe) |
| Market opportunity | $1.5 billion |
| US hospitalizations annually | >50,000 |
The human cost is devastating. Michelle Dinneen, whose daughter Sophia was diagnosed with UCD at birth, described a life of constant vigilance—weighing every gram of food, emergency room visits for ammonia checks, and ultimately a liver transplant at age five.
"The improved ammonia control would have been the ultimate factor in giving us some peace of mind," Dinneen said.
Hepatic Encephalopathy
| Metric | Value |
|---|---|
| Severe recurring HE patients | 80,000 (US) + 150,000 (Europe) |
| Market opportunity | >$2 billion |
| HE-related hospitalization costs | >$75,000 per visit |
| Annual US healthcare burden | >$10 billion |
Current therapy with rifaximin doesn't directly lower ammonia—Scharschmidt's own observational study showed 27% of patients experienced additional HE events despite treatment, with 85% resulting in hospitalization.
Financial Position and Timeline
Korro ended Q3 2025 with $102.5 million in cash, with runway extending into the second half of 2027. The November restructuring—while painful—positioned the company to reach key clinical milestones.
| Milestone | Expected Timing |
|---|---|
| Regulatory filing (IND/CTA) | H2 2026 |
| First-in-human study | 2027 |
| Cash runway | Through H2 2027 |
| GalNAc AATD candidate nomination | H1 2026 |
What Investors Should Watch
Clinical trial design clarity: Management indicated the first goal is to demonstrate ammonia lowering in humans, but specific trial design awaits regulatory alignment. The company is seeking feedback from multiple regulatory bodies given the rare disease populations.
Dosing frequency: Preclinical data supports every-other-week subcutaneous dosing, a major improvement over three-times-daily oral Ravicti. Confirmation of this regimen in humans would be significant.
Novo Nordisk partnership: The 12-month pause on Korro's collaboration with Novo remains in effect. Any resumption of that relationship would signal external validation of the platform.
Competitive dynamics: Wave Life Sciences and AiRNA are pursuing RNA editing for AATD with GalNAc delivery. Korro's pivot to hyperammonemia represents a strategic differentiation, but the company still plans to develop a GalNAc AATD candidate.
The Bottom Line
Korro Bio is attempting something genuinely novel: using RNA editing not to fix mutations, but to create enhanced protein variants that bypass broken biological pathways. The preclinical data is compelling—robust ammonia lowering in multiple disease models with clean safety signals and efficient liver targeting.
But this is still a preclinical story in a company that just experienced a devastating clinical failure. The path from humanized mice to human patients is littered with biotechs that couldn't make the translation.
What Korro has in its favor: deep disease expertise from Scharschmidt, a mechanistically differentiated approach that requires far less editing efficiency than mutation correction, a sizable and underserved market, and sufficient cash to reach clinical proof-of-concept.
What remains to be proven: that the preclinical efficacy translates to humans, that the dosing regimen is truly convenient, and that the company can execute without another setback.
For a stock trading at $10.50—down from $55 just three months ago—the risk-reward may be compelling for investors willing to bet on a second act.
Korro Bio held its virtual Analyst Day on January 27, 2026. The event featured CEO Ram Aiyar, CSO Loïc Vincent, COO Todd Chappell, hepatologist Bruce Scharschmidt MD, and UCD caregiver Michelle Dinneen.
Related Companies