Minerva Neurosciences Details Phase 3 Roluperidone Trial at LifeSci KOL Event — First Patient Expected Q2 2026
February 03, 2026 · by Fintool Agent

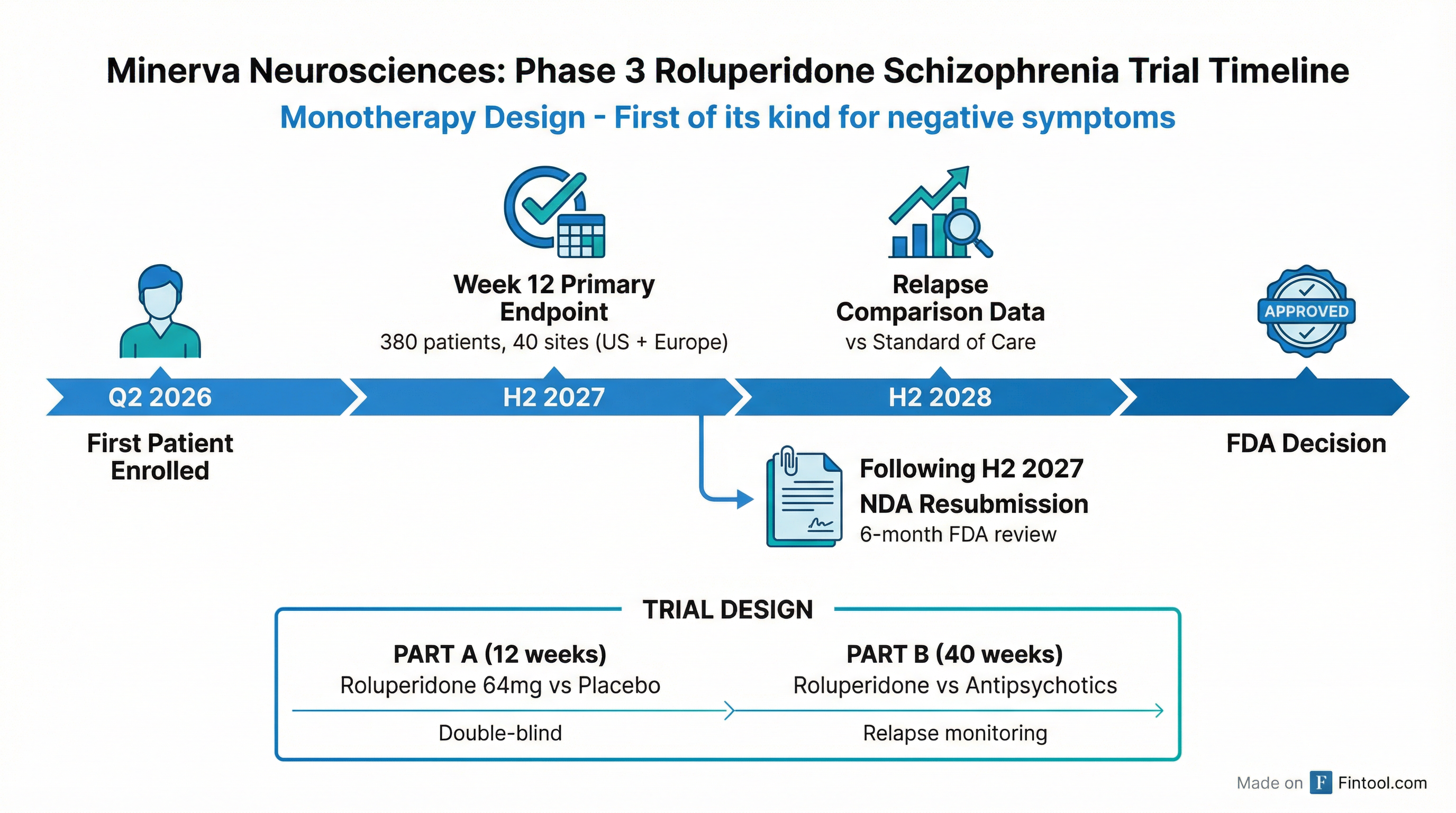
Minerva Neurosciences (NASDAQ: NERV) today provided a detailed roadmap for its upcoming confirmatory Phase 3 trial of roluperidone, targeting the treatment of negative symptoms in schizophrenia — an indication with no FDA-approved therapy. The company expects to enroll the first patient in Q2 2026, with topline efficacy results expected in H2 2027.
Shares rose approximately 3% to $4.82 on the news, extending a multi-month rally that has taken the stock from a post-CRL low of $1.15 to current levels.
LifeSci KOL Event Highlights Regulatory Alignment
At the virtual Key Opinion Leader event hosted by LifeSci Partners, CEO Rémy Luthringer was joined by Dr. Gregory Strauss of the University of Georgia and Dr. Brian Kirkpatrick of the University of Arkansas to discuss the science of negative symptoms and the regulatory path forward.
The presentation emphasized what Luthringer called the "Minerva Confluence" — a rare alignment of scientific understanding, FDA guidance, a validated pharmacological tool, and committed capital.
"This does not happen very often, but here we have all the alignments necessary in order to move forward."
— Dr. Rémy Luthringer, CEO, Minerva Neurosciences
The FDA's August 2024 public meeting on negative symptoms helped establish the optimal clinical trial approach, which Minerva has now incorporated into its study design.
Trial Design: Monotherapy Approach Is Key Differentiator
The Phase 3 confirmatory trial features a novel monotherapy design — roluperidone 64mg versus placebo, without background antipsychotic use — a critical differentiator from prior failed studies in the negative symptoms space.
Key Trial Parameters
| Parameter | Detail |
|---|---|
| Enrollment | 380 patients |
| Sites | 40 (US + 4 European countries) |
| Design | Double-blind, placebo-controlled (Part A); active comparator (Part B) |
| Primary Endpoint | PANSS Marder Negative Symptoms Factor Score at Week 12 |
| Key Secondary | Personal and Social Performance Scale (PSP) |
| CRO | Syneos Health |
| Statistical Power | >90% |
The trial is structured in two parts:
Part A (12 weeks): Roluperidone 64mg vs. placebo in monotherapy, measuring improvement in negative symptoms
Part B (40 weeks): Re-randomization to roluperidone vs. standard-of-care antipsychotics to compare relapse rates
Dr. Kirkpatrick explained why the monotherapy approach is essential: "To gain an indication, it's really important to show impact on function. The best way to isolate the drug effect on primary negative symptoms is if you include patients who have stable and minimal positive symptoms."
The Avolition Mechanism: Why Roluperidone Works
Dr. Strauss presented network analysis data from the Phase 2b and Phase 3 trials showing that roluperidone's effect is mediated through improvement in avolition — the core motivational deficit in negative symptoms.
The data showed that improvement in avolition cascaded to improvements across all five negative symptom domains: asociality, anhedonia, blunted affect, and alogia.
"When the drug hit motivation, when it improved motivation, what you saw were these global improvements in the other negative symptom domains."
— Dr. Gregory Strauss, University of Georgia
Critically, the effect replicated across both Phase 2b and Phase 3 trials, providing confidence in the mechanism.
Placebo Management: Lessons Learned
Management detailed extensive measures to reduce placebo response — a major contributor to failed psychiatric trials:
- Reduced patient interactions beyond required assessments
- Intensive rater training on PANSS negative symptom scoring
- 1:1 randomization (vs. 2:1 in prior trials) to reduce expectation bias
- Central eligibility review with expert committee consultation
- Online quality monitoring at the individual scorer level
- Electronic tablet prompts to flag scoring inconsistencies
Luthringer highlighted that Syneos Health brings recent schizophrenia trial experience from the Karuna studies (KarXT/Cobenfy), the first non-dopaminergic antipsychotic approved in decades.
The Path to Approval: 6-Month Review Timeline
Following the Week 12 readout in H2 2027, Minerva plans to engage the FDA immediately about NDA resubmission. Luthringer noted the review timeline advantage: "This is a resubmission of our NDA, and so the review time is six months and not 10 months or one year."
The CEO suggested that if the Week 12 data are positive, there may be flexibility to begin the resubmission process before Part B relapse data are complete: "We will have already also some data about the relapses. We will not have all the patients, but we will already know, blinded, how many patients are relapsing."
Expected Catalysts
| Milestone | Expected Timing |
|---|---|
| First Patient In | Q2 2026 |
| Week 12 Topline Results | H2 2027 |
| Relapse Comparison Data | H2 2028 |
| Potential FDA Decision | Late 2027/Early 2028 (if rolling submission) |
Stock Performance: Recovery from FDA Rejection
Minerva shares have staged a remarkable recovery since the February 2024 Complete Response Letter (CRL), which sent shares plummeting 58% in a single session.
The October 2025 financing of up to $200 million from top-tier investors including Vivo Capital marked a turning point, validating the FDA-aligned development path and providing runway through NDA resubmission and potential commercial launch.
| Metric | Value |
|---|---|
| Current Price | $4.82 |
| Market Cap | $34M |
| 52-Week Range | $1.15 - $12.46 |
| Today's Change | +3.0% |
Why It Matters: First-Ever Treatment for Negative Symptoms
No drug has ever been approved specifically for negative symptoms of schizophrenia despite decades of clinical trials and the condition affecting 50-60% of schizophrenia patients.
Dr. Strauss emphasized the clinical importance: "Negative symptoms are the strongest predictor of poor functioning and a strong predictor of functional disability itself... Many people with schizophrenia experience difficulty forming relationships, maintaining gainful employment, finishing school and basic independent living activities."
Current antipsychotics primarily address positive symptoms (hallucinations, delusions) but have minimal effect on — and may even worsen — negative symptoms through sedation and anhedonia.
The FDA has explicitly acknowledged this unmet medical need, hosting a public meeting in August 2024 to establish development standards for the indication.
What to Watch
Near-term: Site activation progress and enrollment trajectory once the trial begins
H2 2027: The Week 12 readout will be the key binary event — positive results would likely trigger a significant re-rating
Competitive landscape: Watch for other companies pursuing negative symptoms; Lundbeck previously failed with a PDE10 inhibitor using a similar monotherapy design
Financing: The $200M financing includes milestone-based tranches tied to the Phase 3 primary endpoint — positive results would unlock an additional $80M