Neuralink to Begin 'High-Volume Production' of Brain Chips in 2026, Musk Says
January 1, 2026 · by Fintool Agent
Elon Musk's brain-computer interface company Neuralink is preparing to dramatically scale its operations, with the billionaire announcing that the company will begin "high-volume production" of brain implants and transition to "almost entirely automated" surgical procedures in 2026.
The announcement, made on X on New Year's Eve, marks a pivotal shift from experimental technology to commercial-scale medical device manufacturing—a transition that could reshape treatment options for millions of patients with paralysis and neurological disorders.
"Device threads will go through the dura, without the need to remove it," Musk wrote, referring to the tough outer membrane protecting the brain. "This is a big deal."
From Lab to Scale
The planned manufacturing ramp-up comes after a year of significant milestones for the nine-year-old company.
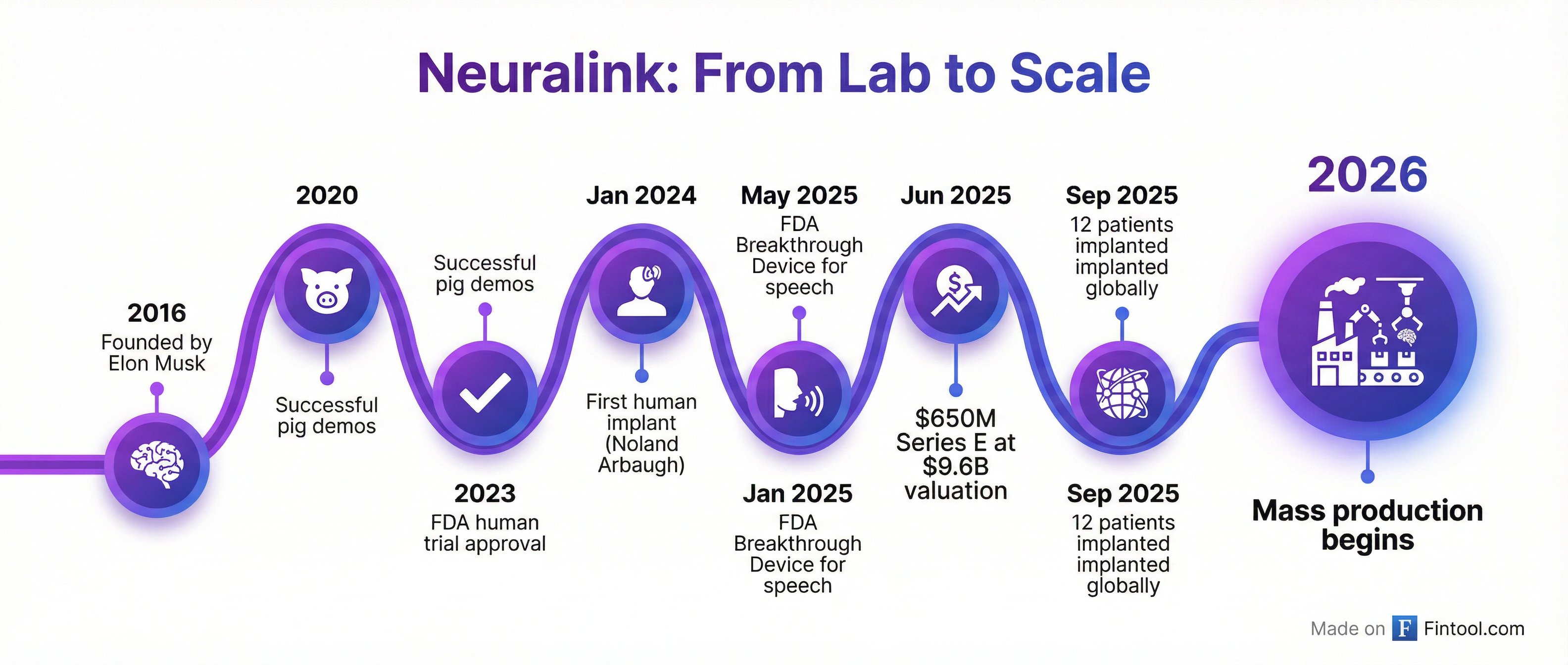
Neuralink secured $650 million in Series E funding in June 2025, valuing the company at approximately $9.65 billion pre-money. The round attracted heavyweight investors including ARK Invest, Founders Fund, Sequoia Capital, Thrive Capital, G42, Lightspeed, and Qatar Investment Authority.
The funding has fueled rapid clinical expansion. As of September 2025, twelve patients worldwide had received Neuralink's N1 implant and were actively using it to control digital devices with their thoughts.
How the Technology Works
The Neuralink N1 implant is roughly the size of a coin and replaces a small piece of skull bone. From the chip, 64 ultra-thin threads—each about 20 times thinner than a human hair—fan out into the patient's brain, carrying 1,024 electrodes that detect and decode neural signals.
Currently, implanting the device requires a human surgeon to remove a portion of the patient's skull before a robotic arm takes over to insert the chip. Musk's announcement suggests Neuralink is developing techniques to place the threads through the dura without removing it—a less invasive approach that could reduce surgical risk and recovery time.
The company's first patient, Noland Arbaugh, a quadriplegic who received his implant in January 2024, has demonstrated the technology's capabilities in public livestreams, using thought alone to play video games, browse the internet, and control a computer cursor. He has achieved information transfer rates exceeding nine bits per second—double the previous brain-interface record.
Expanding Clinical Applications
Beyond basic computer control, Neuralink has secured FDA Breakthrough Device Designation for both vision and speech restoration programs, potentially opening pathways to help blind individuals see and those with severe speech impairments communicate.
The company has also launched the CONVOY trial to test connecting its brain chip to robotic arms—a step toward restoring physical movement, not just digital control.
Clinical trials are now underway at leading neurosurgical centers across three countries and two continents:
- Barrow Neurological Institute (Phoenix, Arizona)
- The Miami Project to Cure Paralysis at University of Miami
- University Health Network at Toronto Western Hospital (Canada)
- Cleveland Clinic Abu Dhabi (UAE)
The Competitive Landscape
Neuralink isn't alone in the race to commercialize brain-computer interfaces. The global BCI market is projected to grow from $3.21 billion in 2025 to $12.87 billion by 2034, representing a 16.7% compound annual growth rate.
Several competitors have taken different technological approaches:
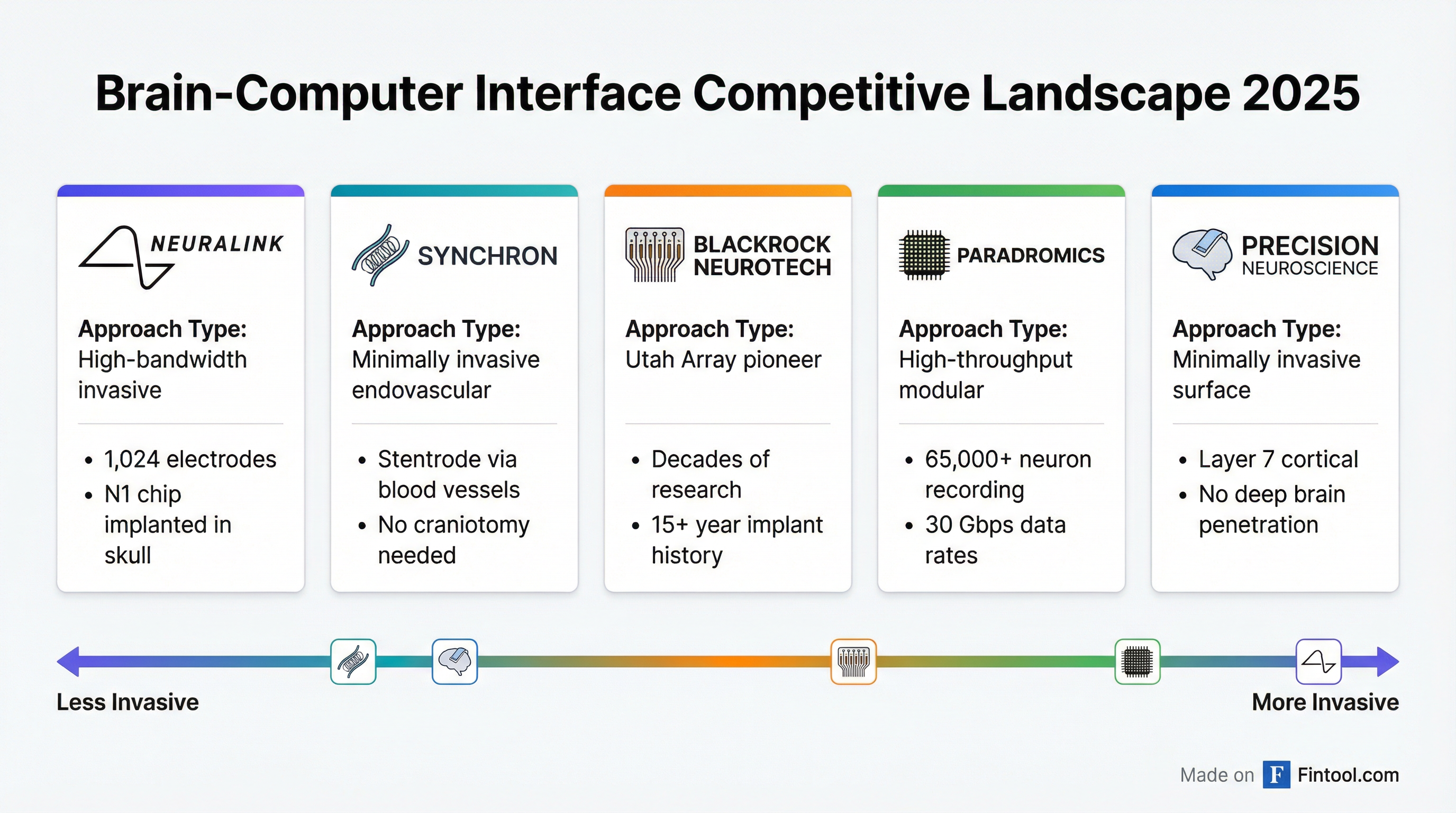
Synchron, backed by Bill Gates, Jeff Bezos, and ARCH Venture Partners, has developed the Stentrode—a device inserted through blood vessels that avoids open brain surgery entirely. The company has demonstrated patients controlling iPads and iPhones through thought and recently partnered with Apple to integrate with its device ecosystem.
Blackrock Neurotech, the industry pioneer, has decades of experience with its Utah Array technology. The company's implants have been used experimentally in dozens of patients and remain the gold standard for high-fidelity neural recording.
Precision Neuroscience, co-founded by Neuralink's former co-founder Benjamin Rapoport, has developed the Layer 7 cortical interface—a thin, flexible electrode array that sits on the brain's surface without penetrating tissue. The company has raised over $155 million and positions itself as a safer, more scalable alternative.
Paradromics boasts the highest-bandwidth system, capable of recording from over 65,000 neurons simultaneously with 30 Gbps data rates. The company has received FDA Breakthrough Device designation and strategic backing from NEOM in Saudi Arabia.
What Mass Production Means
Musk has previously suggested Neuralink could have over 1,000 patients with implants by 2026, and in November 2024, the company went on a hiring spree for manufacturing technicians and microfabrication specialists.
The shift to automated surgery is particularly significant. Current procedures require highly specialized neurosurgeons and operating rooms, limiting how many implants can be performed. An automated system could theoretically allow faster, more consistent procedures at a greater number of medical centers.
The addressable market is substantial. In the United States alone, approximately 5.4 million people live with paralysis that impairs their ability to use computers or communicate.
Challenges Ahead
Despite the optimistic timeline, significant hurdles remain. Clinical trials for novel medical devices typically require years of data collection, with patients staged months apart to monitor for problems. Neuralink's current U.S. trial has space for just five volunteers, and its Canadian trial has room for six.
Software calibration also presents ongoing challenges. Arbaugh has noted that his "model"—the mapping of imagined movements to cursor actions—degrades over hours and days, requiring as long as 45 minutes of retraining tasks to recalibrate. Neuralink's software team is working to reduce this to just a few minutes.
And while Neuralink has achieved impressive technical milestones, it has yet to receive FDA approval for commercial sale. The path from clinical trial to market typically takes years, though the Breakthrough Device designation could accelerate review.
What to Watch
With $1.2 billion raised to date and a $9.65 billion valuation, Neuralink has the capital to pursue its ambitious manufacturing plans. The key milestones to monitor in 2026:
- Patient volume: Can Neuralink scale from 12 implants to the hundreds Musk has projected?
- Automated surgery rollout: When and where will the new streamlined procedures debut?
- FDA progress: Will the company advance toward commercial approval for its paralysis indication?
- Competitive developments: How will Synchron, Precision, and others respond?
For the millions of patients with paralysis, ALS, and severe neurological conditions, the answer to these questions could determine whether brain-computer interfaces become a viable treatment option—or remain an expensive experiment. Musk is betting 2026 is the year the technology breaks through.
Related