Five Quince Therapeutics Directors Resign One Day After 91% Stock Crash
February 2, 2026 · by Fintool Agent

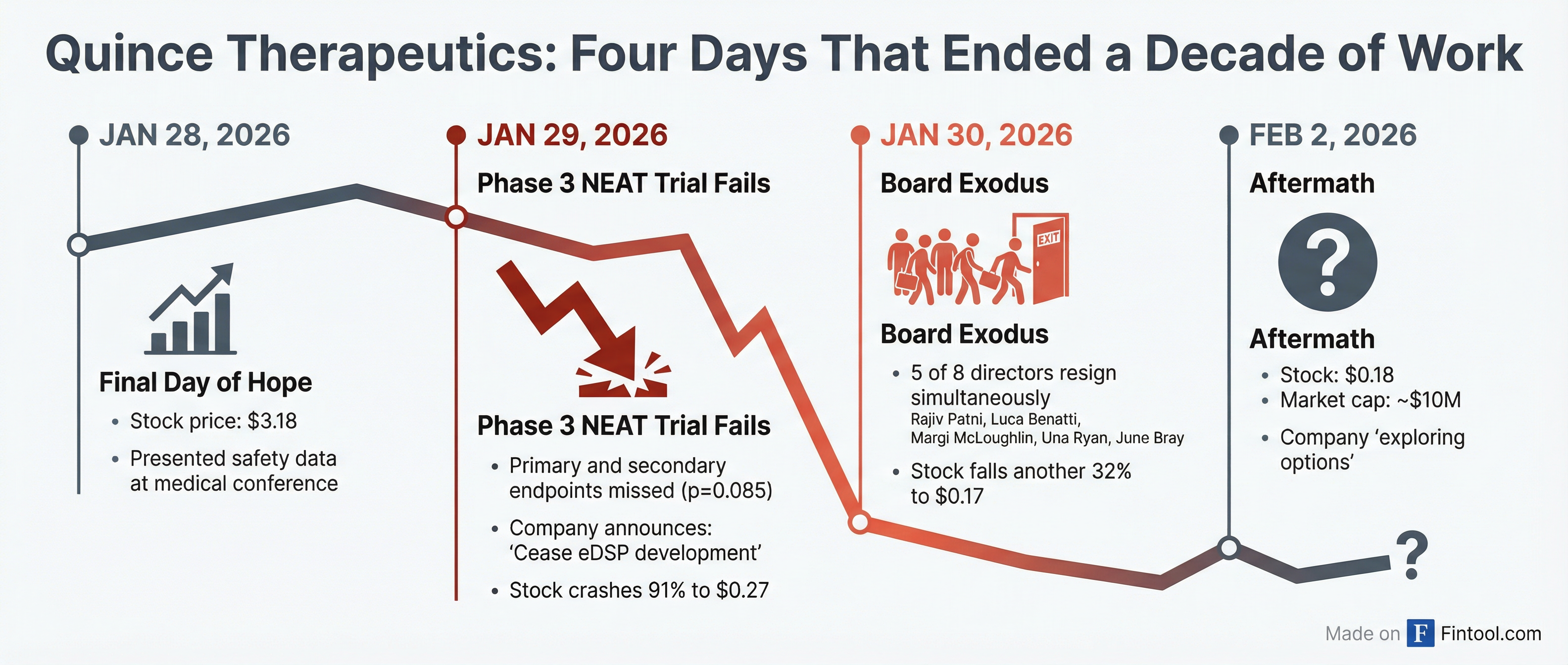
Five of Quince Therapeutics'+5.88% eight board members resigned simultaneously on January 30, 2026—just one day after the company's pivotal Phase 3 trial failed and its stock crashed 91%.
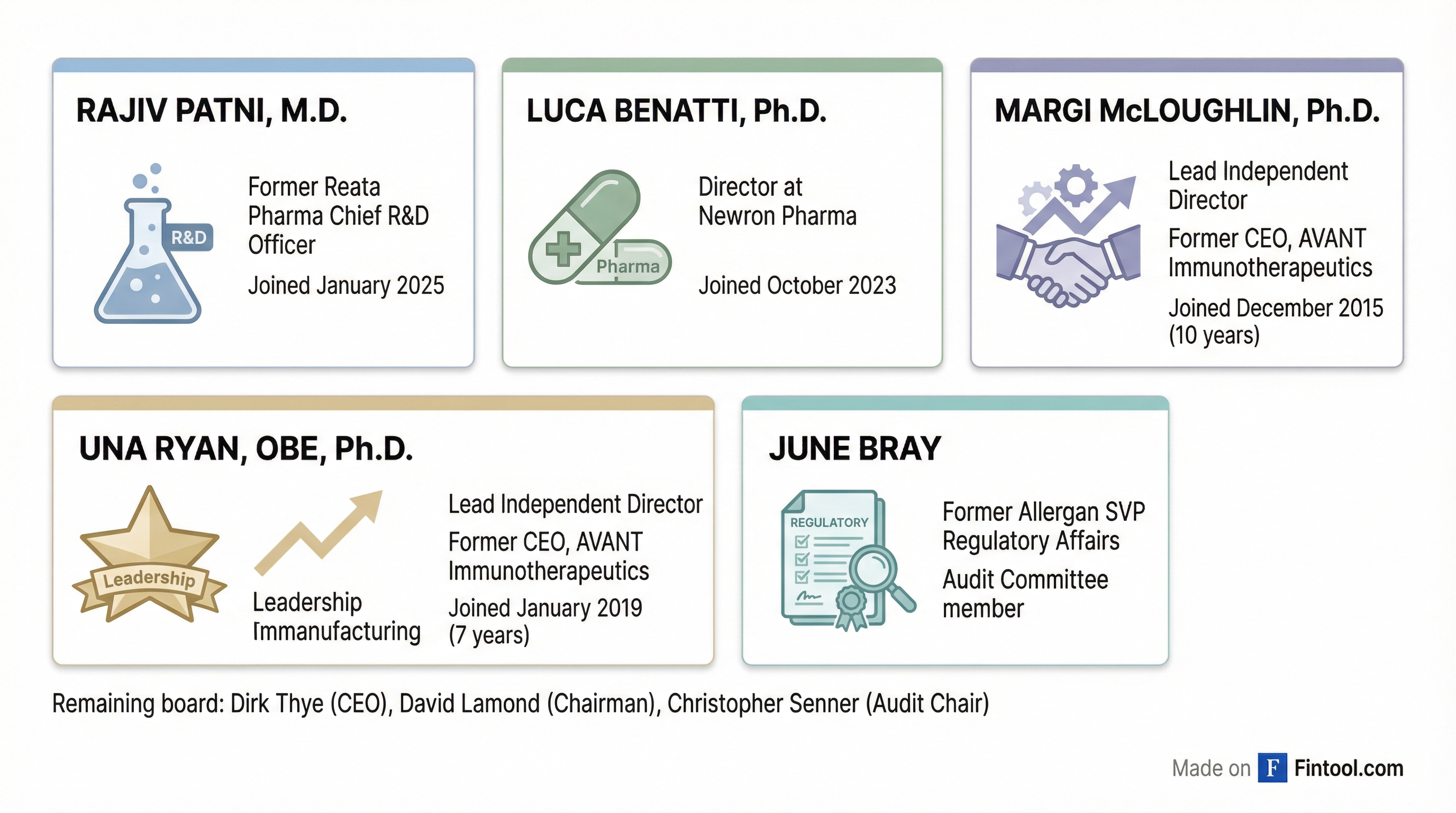
The mass departure leaves the South San Francisco biotech with the minimum three directors required for a public company board: CEO Dirk Thye, Chairman David Lamond, and audit committee chair Christopher Senner. The exodus signals the effective end of Quince as an operating biotech company, following a decade of work on its lead asset.
The Four-Day Collapse
On January 28, 2026, Quince presented safety data from its lead program at a medical conference, with shares trading at $3.18. Twenty-four hours later, everything changed.
The company announced on January 29 that its pivotal Phase 3 NEAT trial of eDSP in Ataxia-Telangiectasia (A-T) failed to meet its primary endpoint, which measured neurological improvement using the RmICARS scale. The p-value of 0.0851 missed the threshold for statistical significance. The key secondary endpoint also failed (p=0.522).
"We express our compassion and hope for future therapeutic options to the A-T community," CEO Dirk Thye said in the announcement. "We have tremendous gratitude toward the patients, their families, academic investigators and study sites, as well as all Quince employees, who worked so diligently over many years on this program."
The stock collapsed 91.4% that day—from $3.14 to $0.27.
The Board Exodus
The following day, January 30, five directors submitted their resignations "effective immediately." The departing board members represented the bulk of the company's independent oversight and specialized expertise:
The 8-K filing contains the standard boilerplate that "such resignations were not the result of any disagreement between such director and the Company on any matter relating to the Company's operations, policies, or practices." In biotech, this language often accompanies situations where continued board service offers little upside and potential liability exposure as a company winds down.
The stock fell another 32% on the resignation news, closing at $0.17.
Financial Reality: Going Concern Warning
The board departures come against a backdrop of severe financial distress. In its most recent 10-Q, Quince carried a going concern warning, acknowledging "substantial doubt exists with respect to the Company's ability to continue as a going concern within one year."
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Cash & Equivalents | $6.2M | $7.8M | $16.8M | $6.5M |
| Total Debt | $14.8M | $15.8M | $17.5M | $18.0M |
| Net Loss | -$12.5M | -$15.0M | -$16.0M | -$13.4M |
| Revenue | $0 | $0 | $0 | $0 |
The company has no revenue, burns approximately $13-16 million per quarter, and carries an $11.7 million debt obligation to the European Investment Bank maturing in August 2026. A minimum cash covenant was already waived through Q1 2026 due to the company's inability to maintain required balances.
From 52-Week High to Penny Stock in 52 Days
The collapse erased over $250 million in market capitalization. QNCX hit its 52-week high of $4.55 on December 12, 2025, when investor optimism around the Phase 3 readout peaked. Fifty-two days later, the stock trades at $0.18—a 96% decline.
The current market cap of approximately $10 million is a fraction of the $26.3 million in cash and investments the company reported as of September 30, 2025. This discount reflects investor skepticism about recovery prospects and the likelihood of further dilution or value destruction in any restructuring scenario.
What 'Exploring Options' Typically Means
The company's statement that it "intends to preserve cash and explore available options" is biotech shorthand for several possible outcomes:
-
Reverse merger: A private company acquires Quince's public shell and Nasdaq listing, providing liquidity to existing shareholders (often at a steep discount)
-
Asset sale: The AIDE technology platform or remaining IP could be sold to a larger pharma company, though value is limited given the clinical failure
-
Strategic partnership: Unlikely given the failure, but a partner could potentially fund exploration of eDSP in a narrower patient subgroup
-
Orderly wind-down: Liquidate remaining assets, pay creditors, and distribute any residual value to shareholders
Given the going concern warning, debt obligations, and absence of any viable pipeline, a reverse merger or orderly wind-down appears most likely. The minimal remaining board—essentially the CEO, a chairman with significant ownership, and an audit chair—is consistent with a caretaker structure for managing such transactions.
The Bigger Picture: A-T Patients Left Without Options
Ataxia-Telangiectasia is a rare, inherited neurodegenerative disorder with no approved treatments in any global market. Approximately 4,600 diagnosed patients in the U.S. suffer from progressive loss of coordination, weakened immune systems, and elevated cancer risk, with a median lifespan of 25-30 years.
The NEAT trial enrolled 105 participants across the U.S., U.K., and Europe. Despite the failure, 104 of 105 participants elected to continue in the company's open-label extension study, reflecting the desperation of a patient community with no alternatives.
What to Watch
- Q4 2025 earnings and cash update: Will reveal actual runway and any strategic announcements
- Reverse merger announcements: Common outcome for biotechs in this situation
- EIB debt maturity (August 2026): Looming deadline that constrains options
- Nasdaq compliance: At $0.18, the stock is well below the $1 minimum bid price requirement; delisting risk rises if not addressed
Related Companies