Ultragenyx Crashes 44% After Setrusumab Fails Two Phase 3 Trials
December 29, 2025 · by Fintool Agent
Ultragenyx Pharmaceutical shares collapsed 44% to $19.11 on Monday after the company's experimental bone disease drug setrusumab failed to meet primary endpoints in two Phase 3 clinical trials. Partner Mereo Biopharma was hit even harder, plunging 90% to $0.22 as the failure devastated the smaller company's pipeline.
The double failure marks a significant setback for patients with osteogenesis imperfecta (OI), a rare genetic disorder that causes brittle bones and frequent fractures. Approximately 60,000 patients in commercially accessible regions have no approved treatment options.
The Trial Results
Neither the Orbit study (adults and young adults aged 5-25) nor the Cosmic study (pediatric patients aged 2-7) achieved statistical significance against their primary endpoints of reducing annualized clinical fracture rates.
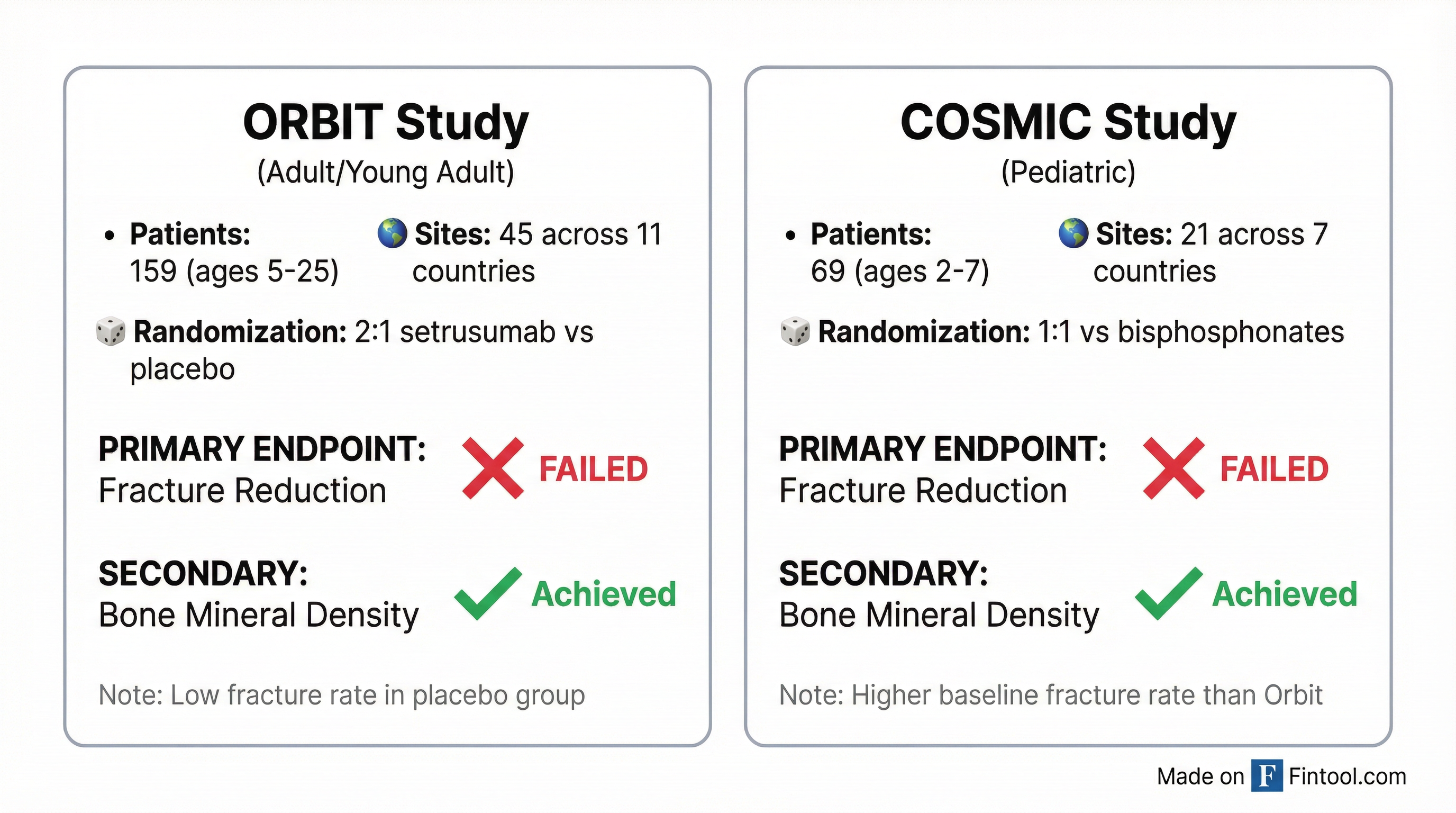
The disconnect between bone density improvement and fracture reduction confounded management. In the Orbit study, setrusumab produced "statistically significant and substantial improvements in BMD compared to placebo"—yet fractures didn't follow. Adding to the puzzle: the placebo group experienced an unexpectedly low fracture rate.
The pediatric Cosmic study showed more encouraging trends. Patients had higher baseline fracture rates, and setrusumab demonstrated a reduction in fractures versus bisphosphonates—but the effect failed to reach statistical significance.
Management's Confidence Didn't Match Reality
The failure is particularly striking given management's bullish messaging throughout 2025. On the Q1 2025 earnings call, Chief Medical Officer Eric Crombez stated: "Based on the Phase II data we previously shared, we are confident that the study will show a clinically and statistically significant reduction in annualized fracture rate at either the second interim or final analysis."
CEO Emil Kakkis had been even more emphatic during the Q4 2024 call: "We feel confident one way or another the trial is going to end and we'll be successful this year."
The science seemed sound. In a December 2024 conference, Kakkis explained how setrusumab represented a "paradigm shift" in understanding OI—that the problem wasn't just defective collagen but inadequate bone production. Mouse models showed anti-sclerostin antibodies could normalize bone strength. Phase 2 data showed a 67% reduction in fracture rate.
Yet Phase 3 didn't replicate those results. The disconnect appears to stem from the placebo arm performing unexpectedly well, making it harder to demonstrate a treatment effect—a common challenge in rare disease trials with heterogeneous patient populations.
Market Reaction
Ultragenyx's market cap collapsed from roughly $3.3 billion to approximately $1.8 billion in a single session. The stock hit a new 52-week low of $18.41 intraday before recovering slightly.
Mereo BioPharma's devastation was more complete. The UK-based company fell from $2.31 to $0.22—a 90% single-day decline that leaves the company with a market cap under $36 million. With just $48.7 million in cash as of Q3 2025, Mereo must now "tightly control costs" while seeking "partnering discussions for alvelestat," its remaining lead asset for alpha-1 antitrypsin deficiency.
| Metric | RARE | MREO |
|---|---|---|
| Prior Close | $34.19 | $2.31 |
| Current Price | $19.11 | $0.22 |
| Change | -44% | -90% |
| Market Cap (Post-Drop) | $1.8B | $36M |
| 52-Week High | $46.50 | $3.84 |
What Remains in the Pipeline
The failure removes setrusumab—which had FDA Breakthrough Therapy, Rare Pediatric Disease, and Fast Track designations—from Ultragenyx's pipeline. But the company maintains it has "four approved products and potentially two near-term gene therapy launches" ahead.
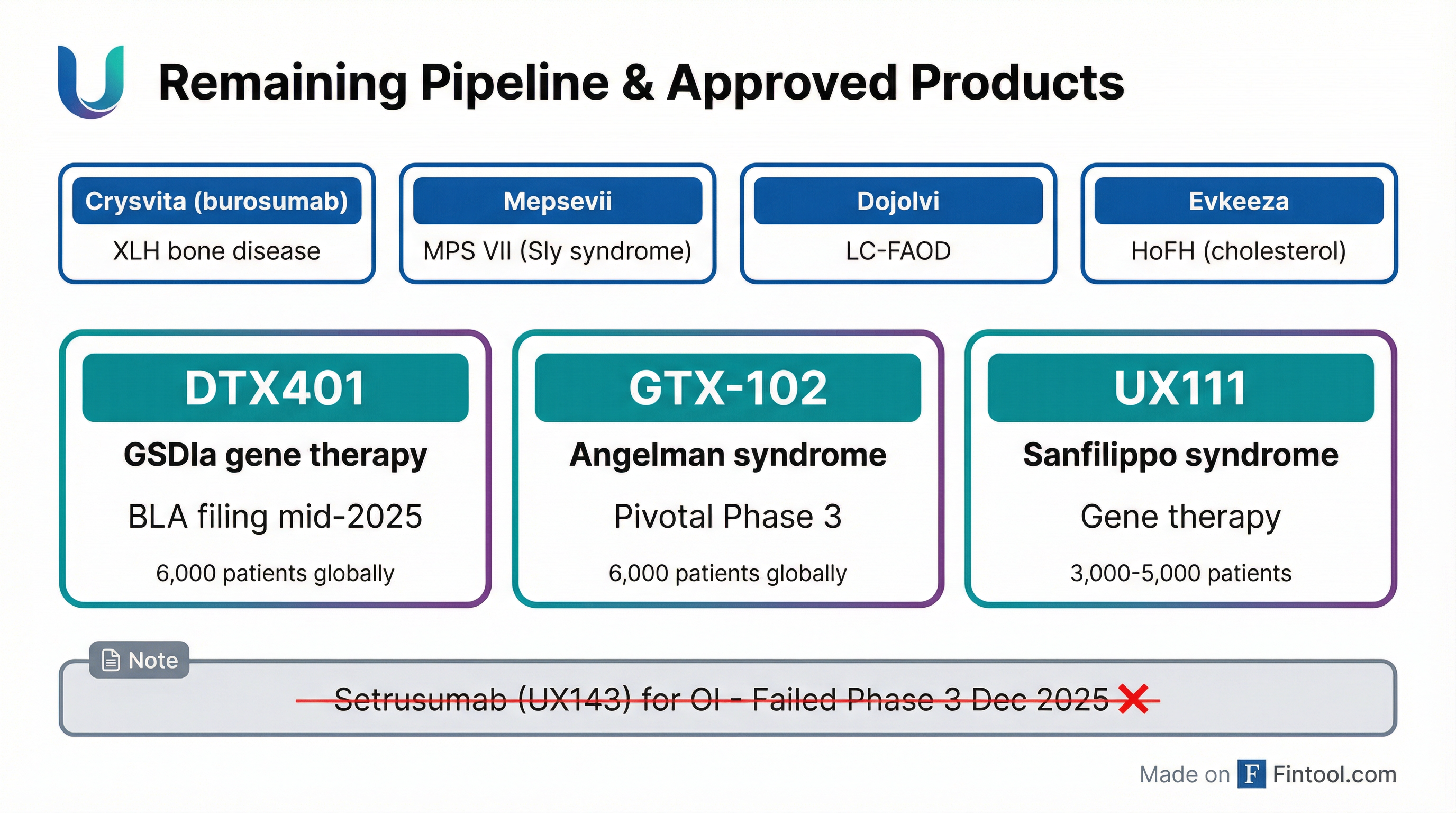
Approved Products:
- Crysvita (burosumab) – X-linked hypophosphatemia
- Mepsevii (vestronidase alfa) – MPS VII (Sly syndrome)
- Dojolvi (triheptanoin) – Long-chain fatty acid oxidation disorders
- Evkeeza (evinacumab) – Homozygous familial hypercholesterolemia
Key Development Programs:
- DTX401 – Gene therapy for Glycogen Storage Disease Type Ia (BLA filing expected)
- GTX-102 – Antisense therapy for Angelman syndrome (pivotal Phase 3)
- UX111 – Gene therapy for Sanfilippo syndrome
Financial Position
The company generated $160 million in revenue in Q3 2025, though it continues to operate at a loss.
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenue | $164.9M | $139.3M | $166.5M | $159.9M |
| Net Income | -$133.4M* | -$151.1M* | -$115.0M* | -$180.4M* |
| Cash | $173.7M | $127.1M | $176.3M | $202.5M |
| Total Debt | $910M* | $890M* | $883M* | $863M* |
*Values retrieved from S&P Global
Ultragenyx announced it will "promptly define and implement significant expense reductions" following the failure, though specifics weren't disclosed.
What to Watch
Despite the setback, Kakkis pointed to the path ahead: "While we are disappointed by these results, we continue to build our commercial revenue from four approved products and prepare for a transformational year ahead with potentially two near-term gene therapy launches and a pivotal Phase 3 readout in Angelman syndrome."
The company is conducting additional analyses on both studies, examining "other bone health and clinical endpoints beyond fractures" to determine next steps—leaving open the possibility that setrusumab's story isn't entirely over.
For Mereo, the calculus is grimmer. With $48.7 million in cash and no near-term revenue, the company's survival may depend on successfully partnering its remaining assets or finding a buyer. The setrusumab collaboration included up to $245 million in potential milestone payments that will now never materialize.
Related: