BRISTOL MYERS SQUIBB (BMY)·Q4 2025 Earnings Summary
Bristol Myers Squibb Beats on Revenue and EPS as Growth Portfolio Hits $26B
February 5, 2026 · by Fintool AI Agent
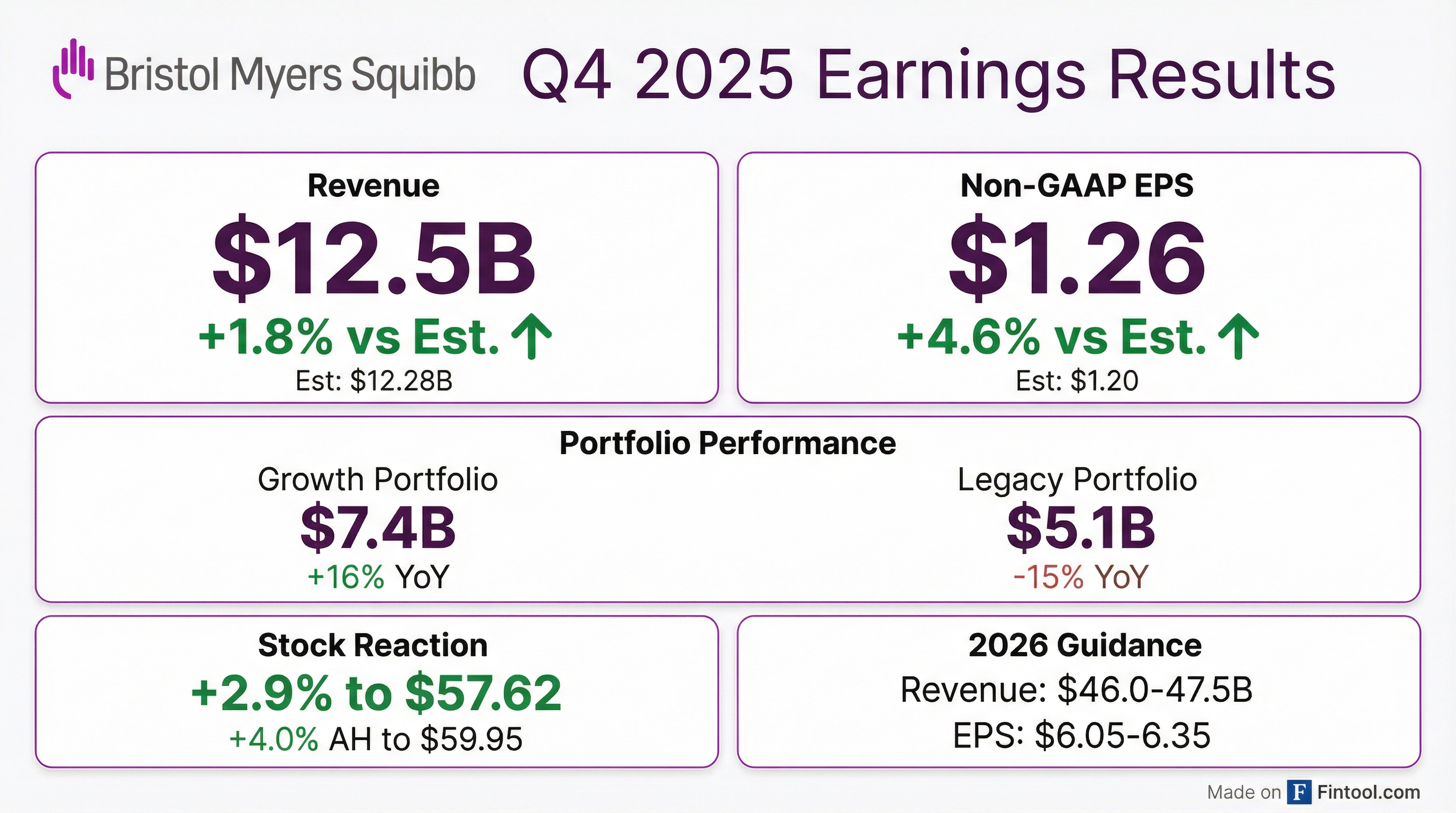
Bristol Myers Squibb delivered a double beat in Q4 2025, with revenue of $12.5B (+1.8% vs consensus) and non-GAAP EPS of $1.26 (+4.6% vs consensus). The Growth Portfolio continued its strong momentum with 16% YoY growth to $7.4B, now representing 55% of total revenues. Full-year 2025 non-GAAP EPS of $6.15 exceeded the $6.11 consensus estimate. The stock rose 2.9% during regular trading and added another 4.0% in after-hours to $59.95 following the results.
Did Bristol Myers Squibb Beat Earnings?
BMY beat on both revenue and EPS in Q4 2025:
Full-year 2025 results also came in ahead of expectations:
Both GAAP and non-GAAP EPS include a $(0.60) per share impact from Acquired IPRD charges and licensing income in Q4, and $(1.40) for the full year.
BMY has beaten EPS estimates in 8 consecutive quarters, with a median beat of ~20%. The streak reflects management's track record of conservative guidance and operational execution during the portfolio transition.*
What Changed From Last Quarter?
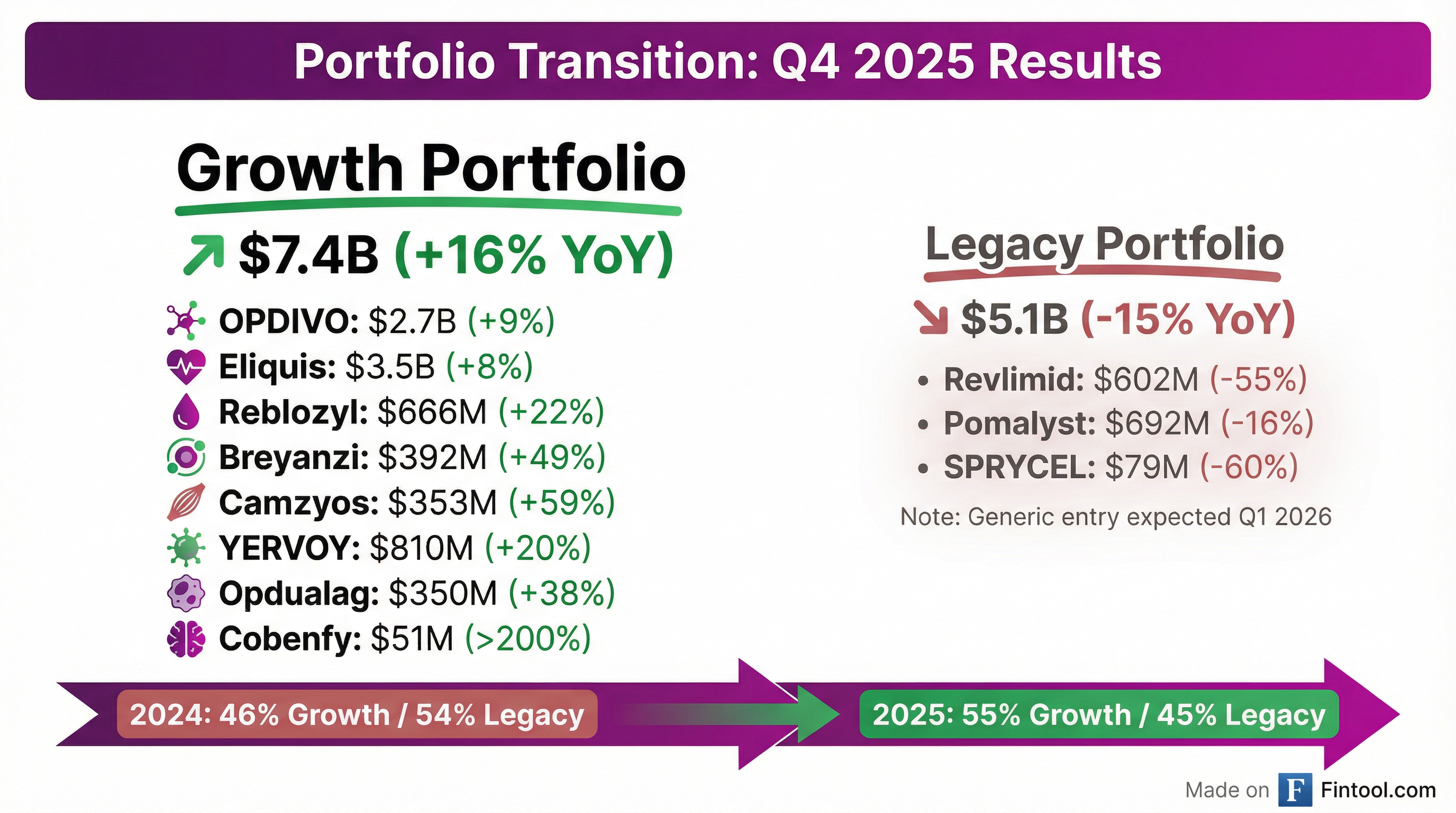
Growth Portfolio acceleration continues. Q4 Growth Portfolio revenues of $7.4B grew 16% YoY (15% Ex-FX), consistent with Q3's +7.5% sequential momentum. Full-year Growth Portfolio revenues reached $26.4B, up 17% YoY, crossing the inflection point where it now exceeds the Legacy Portfolio ($21.8B).
Legacy Portfolio erosion accelerating. Q4 Legacy Portfolio revenues declined 15% YoY to $5.1B as generic competition intensified:
- Revlimid plunged 55% to $602M as volume limits expired in January 2026
- SPRYCEL dropped 60% to $79M
- Pomalyst fell 16% to $692M with generic pomalidomide entry expected Q1 2026
Eliquis remains the anchor. Worldwide Eliquis revenue grew 8% to $3.5B in Q4 (+6% Ex-FX), with U.S. demand growth and market share gains. Management guided 10-15% WW Eliquis growth for 2026.
Margin compression from mix shift. Gross margin of 67.2% (GAAP) / 71.9% (non-GAAP) in Q4 reflected higher Eliquis mix and lower Revlimid/Pomalyst contribution. 2026 gross margin guided at 69-70%.
How Is the Growth Portfolio Performing?
The Growth Portfolio is the key investment thesis for BMY. Here's the Q4 breakdown:
Immuno-Oncology Franchise
Hematology & Cell Therapy
Cardiovascular & Immunology
Neuroscience
What Did Management Guide?
BMY provided 2026 non-GAAP guidance reflecting continued portfolio transition:
Key guidance components:
- Growth Portfolio: Continued strength expected
- Legacy Portfolio: 12-16% decline anticipated
- Eliquis: 10-15% WW revenue growth
- OpEx: Lower YoY from strategic productivity initiative
- Other Income: ~($700M) reflecting diabetes royalties expiration
The $6.05-$6.35 EPS guidance brackets the $6.04 consensus estimate, suggesting modest upside potential if Growth Portfolio momentum continues.
How Did the Stock React?
BMY shares responded positively to the Q4 results:
The stock is trading at 9.4x forward P/E on 2026 consensus EPS of $6.04, compared to large-cap pharma peers at 12-15x. The discount reflects concerns over Legacy Portfolio erosion and Eliquis patent cliff (2028), partially offset by Growth Portfolio momentum and pipeline catalysts.
What Are the Key Catalysts Ahead?
Management highlighted a "data-rich" 2026 with multiple pivotal readouts:
Recent Pipeline Wins:
- Camzyos SCOUT-HCM (January 12): Positive Phase 3 results in adolescents with symptomatic oHCM—met primary endpoint with statistical significance on multiple secondary endpoints and no new safety signals
- Breyanzi MZL approval (December 4): First and only CAR T treatment for relapsed/refractory marginal zone lymphoma after 2+ prior lines
- Breyanzi MCL approval (November 24): European Commission approval for relapsed/refractory mantle cell lymphoma
- Opdivo cHL priority review: FDA assigned April 8, 2026 PDUFA date for 1L Stage III/IV classical Hodgkin Lymphoma
2026 NME Registrational Data:
- Milvexian (anticoagulant) in AF and stroke prevention (LIBREXIA-AF1, LIBREXIA-STROKE1)
- Mezigdomide in RRMM (SUCCESSOR-2)
- Iberdomide in RRMM (EXCALIBER-RRMM)
- RYZ101 in GEP-NETs (ACTION-1)
- Arlo-cel in multiple myeloma (QUINTESSENTIAL)
- Admilparant in IPF (ALOFT-IPF)
2026 LCM Pivotal Data:
- Cobenfy in AD Psychosis (ADEPT-1, 2 & 4)
- Sotyktu in SLE (POETYK SLE-1 & 2)
- Sotyktu PsA PDUFA: March 6, 2026
2026 Early-Stage Data:
- BCMAxGPRC5D dual-targeting CAR T in RRMM
- MYK-224 in HFpEF (AURORA)
- Zola-cel in autoimmune diseases (Breakfree trials)
- Pemidomig + chemo in 1L TNBC (encouraging Phase 2 interim data with BioNTech) — 8 registrational studies expected underway by year-end, including 2 new NSCLC studies now initiating
- Break Free SSC study for Xolocel (CD19 CAR-T) now initiating in systemic sclerosis
- Nabla Metastat (PRMT5 inhibitor) combination data in pancreatic cancer at ESMO next month
CMO Christian Masaccesi characterized 2026 as having "at least 10 pivotal readouts" across hematology, fibrosis, stroke/AFib, and ADCs, calling them "very transformative regimens."
CEO Chris Boerner emphasized execution culture: "Maintaining this strong say-to-do ratio by consistently delivering on our commitments has now been embedded in our culture and will continue to be core to how we operate."
What Are the Key Risks?
Milvexian ACS trial stopped. In November 2025, BMY and Johnson & Johnson announced the decision to stop the Phase 3 Librexia ACS trial evaluating milvexian in patients after acute coronary syndrome events. No new safety concerns were identified, but the trial was discontinued due to efficacy. Importantly, the LIBREXIA-STROKE and LIBREXIA-AF studies continue as planned.
Generic erosion accelerating. The Legacy Portfolio decline is steepening with Revlimid volume limits expired in January 2026 and generic pomalidomide entry expected in Q1 2026. Management guided 12-16% Legacy Portfolio decline for 2026.
Eliquis patent cliff looming. While Eliquis is guided to grow 10-15% in 2026, the patent cliff in 2028 remains a key overhang. Milvexian was positioned as a potential replacement, making pipeline execution critical.
Gross margin compression. Product mix shift toward higher Eliquis and lower Revlimid/Pomalyst is pressuring margins. 2026 gross margin guided at 69-70% vs. 72.6% in 2025.
Capital Allocation Update
Balance sheet strength:
- Cash & investments: ~$11.1B
- Total debt: ~$45.1B
- Achieved $10B debt paydown ahead of schedule
Shareholder returns:
- Quarterly dividend: $0.63/share (17th consecutive annual increase)
- Annual dividend: $2.52/share (~4.4% yield)
- Share repurchase authorization: ~$5B remaining
Business development: Announced Microsoft collaboration in January 2026 to accelerate early lung cancer detection using AI algorithms, expanding access in underserved communities.
Q&A Highlights From the Earnings Call
On Milvexian AFib Trial Confidence (Michael Yee, UBS): Christian Masaccesi (CMO) noted the trial has enrolled over 20,000 patients and the Data Monitoring Committee "regularly continues to endorse the trial progression." He added: "We are well past the point in which OCEANIC AFib was terminated by the DMC because of lack of efficacy... what the DMC is telling us and what we see in a blinded fashion in terms of bleeding rates gives us confidence that we are still very much on target."
On Eliquis 2027 Step-Down Rationale (Courtney Breen, Bernstein): CFO David Elkins clarified the $1.5-2B step-down from 2026 to 2027 is driven by EU patent expirations clustering "late in the year, in the November time frame." The company expects "rapid and steep decline like we have seen with other small molecules outside of the U.S."
On Cobenfy Uptake Trajectory (Geoff Meacham, Citi): Adam Lenkowsky (CCO) reported Cobenfy delivered over 100,000 TRXs since launch, surpassing all schizophrenia analogs. Access is now "virtually 100% across Medicaid and Medicare" and approaching 70% commercial. He noted: "Those physicians who've had a positive experience with Cobenfy have shown an increased propensity to repeat prescribing... We're confident this could be a big drug for the company."
On CELMoD Portfolio Positioning (Jason Gerberry, BofA): Management emphasized CELMoDs (Iberdomide, Mezigdomide) target the 70-80% of multiple myeloma patients treated in the community setting where bispecifics and CAR-T face "safety and accessibility challenges." The goal is to make both drugs "foundational in multiple myeloma, replacing Revlimid in earlier lines of treatment."
On Opdivo Subcutaneous Conversion (Asad Haider, Goldman): Lenkowsky confirmed the launch is tracking well with adoption "across monotherapy indications as well as in the combination setting." BMY remains "very confident in our expectation that physicians will convert 30-40% of the IV business ahead of the LOE."
On Admilparant (LPA-1) Safety Profile (David Risinger, Leerink): Masaccesi addressed hypotension concerns, noting the Phase 3 uses 120mg (double Phase 2 dose) with a run-in to assess safety. "The DMC allowed us to go in Phase 3. In a blinding fashion, we didn't see any rates that raised any concern." Physicians view this as "a very manageable side effect" compared to GI issues plaguing existing therapies.
On Fumitomab Development Scale (Geoff Meacham, Citi): Masaccesi confirmed 7-8 pivotal studies are already starting or in flight across non-small cell lung cancer, gastric, colon, head and neck, and breast cancers. The strategy: "We want to replace where PD-1/PD-L1 inhibitors are playing today... then we want to expand because we believe bringing VEGF on top of PD-L1 inhibition, we can also tackle some of the indications where PD-1/PD-L1 inhibitors are not working well enough."
Bottom Line
Bristol Myers Squibb delivered a clean Q4 beat with the Growth Portfolio now comprising 55% of revenues and growing 17% annually. The portfolio transition thesis is playing out as expected—Legacy erosion from Revlimid/Pomalyst generics is being offset by strength in OPDIVO, Eliquis, Reblozyl, Breyanzi, and Camzyos. The 2026 guidance of $6.05-$6.35 EPS brackets consensus, leaving room for upside if pipeline catalysts deliver. At 9.4x forward P/E, BMY remains attractively valued relative to large-cap pharma peers, with the stock reacting +4% after hours as investors endorsed the transition progress.
Values for consensus estimates and beat percentages retrieved from S&P Global.
Related: