Embecta (EMBC)·Q1 2026 Earnings Summary
embecta Posts EPS Beat as Deleveraging Accelerates, Stock Jumps 4%
February 5, 2026 · by Fintool AI Agent
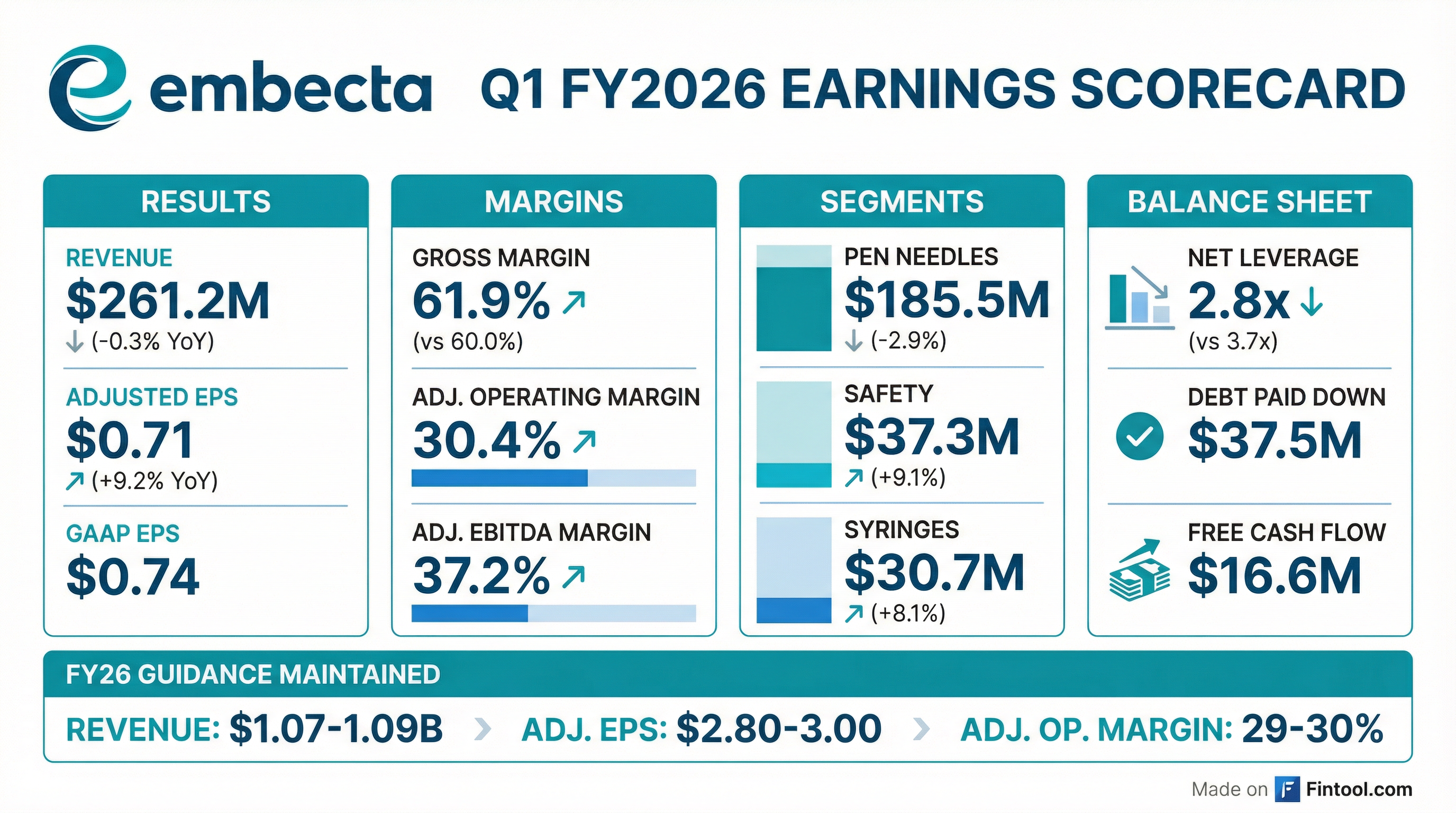
embecta (NASDAQ: EMBC) reported Q1 FY2026 results that beat on earnings while slightly missing on revenue. Adjusted EPS of $0.71 significantly exceeded consensus expectations of $0.46, while revenue of $261.2M came in 1.7% below the $265.7M estimate. The stock jumped 4% on the results as investors rewarded the company's accelerating debt paydown and improving profitability.
Did embecta Beat Earnings?
Adjusted EPS: Beat — $0.71 vs $0.46 consensus (+56%)
Revenue: Slight Miss — $261.2M vs $265.7M consensus (-1.7%)
The dramatic YoY improvement in GAAP EPS (from $0.00 to $0.74) reflects the prior year quarter being weighed down by $39.6M in patch pump discontinuation expenses, which turned into a $9.5M gain this quarter as embecta sold certain assets from the discontinued program.
How Did the Stock React?
EMBC shares rose 4.0% to $11.35 following the earnings release, outperforming the broader market. The stock had declined significantly from its 52-week high of $18.12 to recent lows near $9.20 amid concerns about GLP-1 drugs impacting diabetes injection demand.
The positive reaction suggests investors focused on the EPS beat and deleveraging progress rather than the modest revenue miss.
What Did Management Guide?
FY2026 guidance maintained — no changes from November 2025:
Key Assumptions:
- EUR/USD: ~1.17
- USD/JPY: ~156
- Adjusted Tax Rate: ~23%
- Interest Expense, Net: ~$93M
With Q1 adjusted EPS at $0.71, embecta is on track to hit the low end of the $2.80-3.00 full-year guidance, with potential upside if margin expansion continues.
What Changed This Quarter?
Segment Performance
Key observations:
- Pen Needles weakness continues to be the core headwind, declining 4.4% on a constant currency basis, driven by pricing pressure and volume shifts
- Safety products (+7.3% CC) and Syringes (+5.3% CC) partially offset the pen needle decline, demonstrating portfolio diversification benefits
- Geographic mix: U.S. revenue declined 7.6% to $130.9M while International grew 8.4% (4.6% CC) to $130.3M
Revenue Bridge
Revenue declined $0.7M YoY, driven by:
- Unfavorable pricing: -$7.8M
- Lower contract manufacturing (BD): -$0.7M
- Favorable FX (USD weakness): +$4.6M
- Volume improvement: +$3.2M
Strategic Progress Update
Management provided updates on three strategic pillars:

1. Strengthen Core Business:
- Brand transition advancing in International markets; expect substantially complete by end of calendar year 2026
- Contracted with additional Medicare Part D payer for exclusive access
- Renewed advantaged formulary status with top 3 Medicare Part D payers
- Product design finalized and assembly line equipment installed for market-appropriate pen needles/syringes; manufacturing validation in progress
2. Expand Product Portfolio:
- Collaborating with 30+ pharmaceutical partners across various sales cycle stages for co-packaging pen needles with generic GLP-1 therapies
-
1/3 of partners have selected embecta as supplier and have executed contracts or are in negotiations
- Several partners have signed agreements and placed purchase orders
- Pen needles included in multiple partner-managed regulatory submissions
- Partners anticipating initial generic GLP-1 launches in Canada, Brazil, China, and India in CY2026
- Expanding consumer-friendly Embecta-branded small pack configurations in Canada and select European markets for out-of-pocket GLP-1 users
- Early-stage discussions with branded pharma companies for drugs in development (potential upside beyond $100M opportunity)
3. Increase Financial Flexibility:
- Paid down ~$37.5M of Term Loan B in Q1
- On track to reduce gross debt by ~$150M during FY26
- Net leverage improved to 2.8x from 3.7x YoY
Balance Sheet & Capital Allocation
The significant improvement in net leverage (from 3.7x to 2.8x) reflects both debt paydown and EBITDA improvement. Trailing 12-month adjusted EBITDA increased to $415.2M from $360.3M YoY.
Dividend declared: $0.15 per share, payable March 17, 2026 to shareholders of record February 27, 2026.
Q&A Highlights
On U.S. pricing headwinds: CFO Jake Elguicze clarified that the guidance shift to low end is primarily due to pricing dynamics from a different customer and product mix, not volume weakness. In fact, volumes are now expected to be flattish year-over-year (vs prior expectation of ~1% decline). New product contribution of ~50bps is offsetting the ~50bps contract manufacturing headwind.
On China recovery: CEO Dev Kurdikar explained that the company has reorganized its sales force and introduced a new pen needle that can compete head-to-head with local branded companies. These initiatives are gaining traction, with Q1 performance in line with expectations. Recovery is expected to be more second-half weighted.
On oral GLP-1 impact: Management remains confident in the $100M+ GLP-1 opportunity despite oral launches. Key points:
- Oral GLP-1 launches in 2026 were explicitly included in their $100M estimate
- Early data suggests orals are expanding the market rather than cannibalizing injectables
- Injectables have better weight loss profiles
- Potential oral use cases: needle-averse patients, limited cold storage regions, maintenance therapy
- Most oral prescriptions are going to new-to-therapy patients
On Zepbound KwikPen: Management noted with interest that Lilly's Zepbound received FDA approval for a KwikPen delivery format (vs auto-injector). This represents potential upside—if Zepbound is delivered via pen, patients will need pen needles, and embecta has a strong U.S. position to capitalize.
On pen injector development: The company has started a pen injector development project (not auto-injector). CEO believes they have the R&D and manufacturing capabilities to develop the product, plus established relationships with generic drug companies provide the distribution channel. Still early stages—no timing or market sizing yet.
Revenue cadence: Management now expects 46%/54% first half/second half revenue split (vs 48%/52% in FY25 and prior expectations for similar cadence). The lower first half is driven by U.S. customer mix, competitive intensity, and channel dynamics; the stronger second half reflects improved international outlook.
Risks & Concerns
Tariff exposure: Management highlighted risks from newly instituted tariffs impacting raw materials and products, as well as potential shifts by foreign governments and purchasers toward "local" products.
U.S. pricing pressure: Management is seeing incremental pricing headwinds in the U.S. during the first half due to customer mix changes and competitive intensity. This is the primary driver of guiding to low end of ranges.
GLP-1 headwinds: The continued weakness in pen needles (-4.4% CC) may reflect reduced injection frequency as more patients shift to weekly GLP-1 therapies from daily insulin injections.
Brand transition risk: The ongoing transition from BD branding carries execution risk, though management expects substantial completion by end of CY2026.
Historical EPS Performance
*Values retrieved from S&P Global
Forward Catalysts
- Generic GLP-1 launches — Partners anticipating initial generic GLP-1 launches in Canada, Brazil, China, and India beginning in calendar year 2026, with India approvals already granted to some companies ahead of March 2026 patent expiration
- Zepbound KwikPen launch — Lilly's FDA approval for KwikPen format (expected to launch in coming weeks) represents upside if more Zepbound shifts to pen delivery requiring pen needles
- B2B partnership announcements — With >1/3 of 30+ identified partners contracted or in negotiations, additional deal announcements could validate the portfolio expansion strategy
- Pen injector development — Early-stage pen injector project could expand addressable market long-term
- Brand transition completion — Substantial completion by end of CY2026 removes execution overhang
- Continued deleveraging — Each quarter of debt paydown improves equity value and reduces interest expense
embecta is a pure-play diabetes care company spun off from Becton Dickinson (BDX) in April 2022. The company manufactures pen needles, syringes, and safety devices used by diabetes patients for insulin injection.
Related: EMBC Company Page | Q4 FY2025 Earnings | Earnings Transcript