Immunovant (IMVT)·Q3 2026 Earnings Summary
Immunovant Beats on EPS as D2T RA Trial Fully Enrolls, Stock Jumps 7%
February 6, 2026 · by Fintool AI Agent
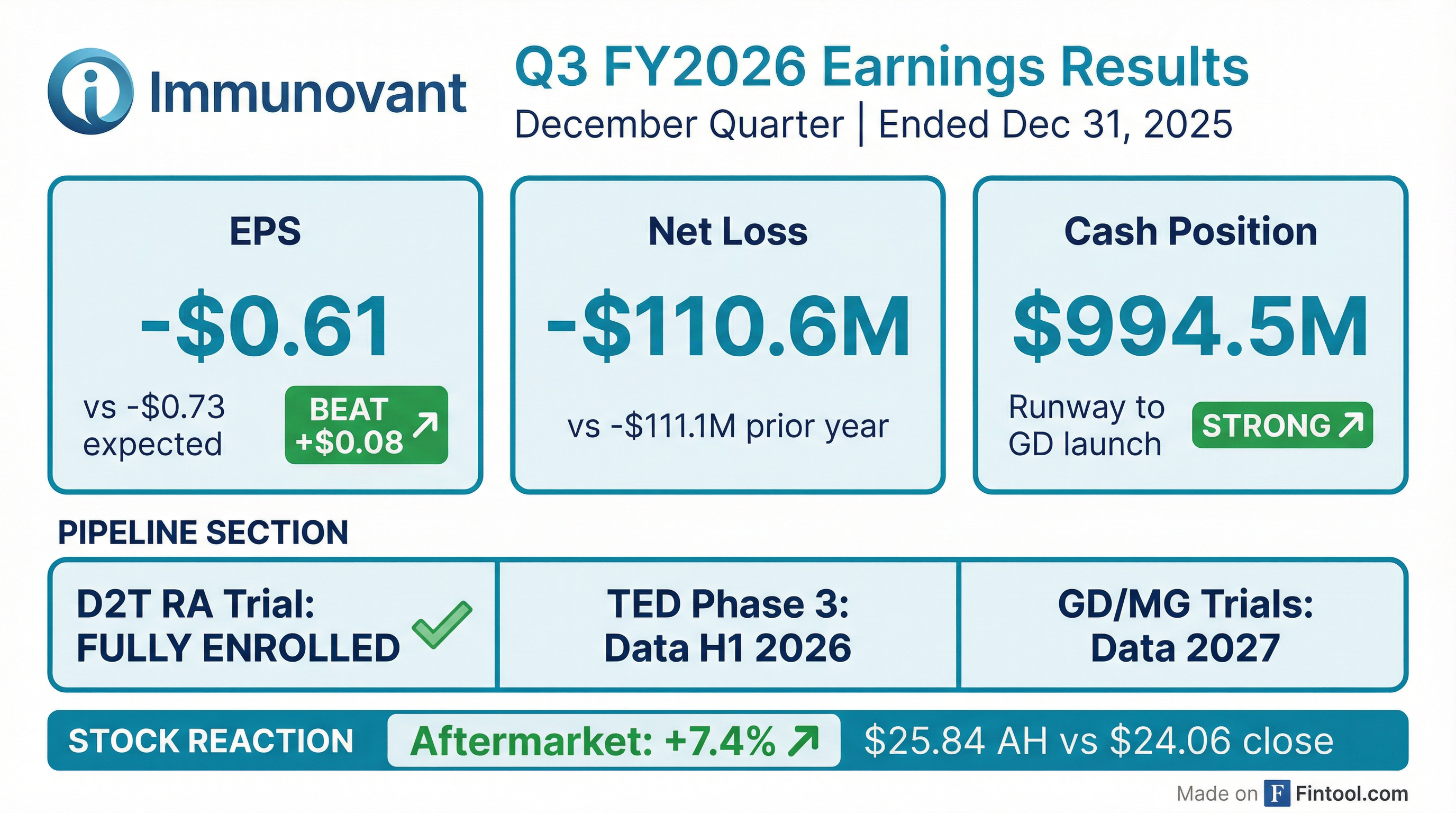
Immunovant (IMVT) reported fiscal Q3 2026 results that beat expectations on EPS while announcing full enrollment of its pivotal D2T RA trial. The clinical-stage biotech posted a narrower-than-expected loss of $0.61 per share versus the Street's $0.73 estimate, driven by disciplined spending as batoclimab trials wind down. Shares rallied 7.4% in after-hours trading to $25.84.
Did Immunovant Beat Earnings?
Yes. Immunovant delivered a meaningful beat on EPS while net loss held essentially flat year-over-year:
The EPS beat was primarily driven by lower G&A expenses (-$4.4M YoY) as the company reduced personnel costs, market research, and IT spending. R&D increased modestly (+$4.4M YoY) as IMVT-1402 clinical trials ramped while batoclimab costs declined.
Beat/Miss History (Last 8 Quarters)
*Values retrieved from S&P Global
Immunovant has beaten estimates 3 of the last 8 quarters, with results improving in recent periods as the company demonstrates better expense management.
What Are the Key Pipeline Updates?
The headline for biotech investors: the D2T RA trial is fully enrolled, putting Immunovant on track for potentially registrational data in H2 2026.
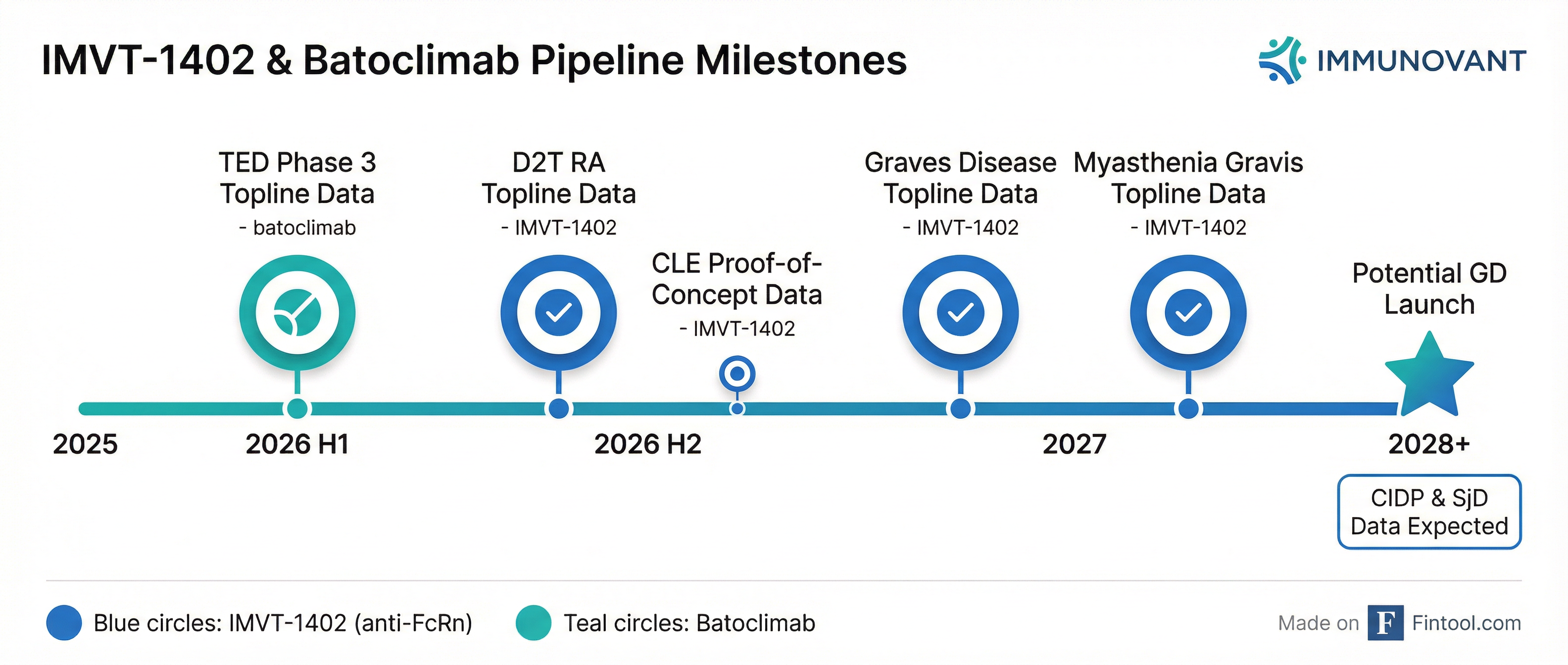
IMVT-1402 (Anti-FcRn)
D2T RA Over-Enrollment: The trial enrolled 170 patients versus the originally anticipated 120, driven by strong enthusiasm from the patient and physician community. Both the open-label period and randomized withdrawal period data will be available in H2 2026.
Batoclimab (First-Gen Anti-FcRn)
Two Phase 3 studies in thyroid eye disease (TED) are expected to report topline data in H1 2026.
How Strong Is the Balance Sheet?
Immunovant ended the quarter with $994.5M in cash, more than doubling from $713.9M at fiscal year-end (March 2025).
The cash boost came from a $550M underwritten offering completed in December 2025 with key institutional investors and Roivant. Management stated this extends runway through the potential commercial launch of IMVT-1402 in Graves' disease, which would be the company's first revenue-generating product.
How Did the Stock React?
Shares closed at $24.06 on Thursday, down 3.1% during regular trading. However, after the earnings release, stock surged 7.4% in after-hours trading to $25.84.
Values retrieved from market data
The positive reaction reflects the EPS beat and, more importantly, the D2T RA full enrollment milestone. Biotech investors value clinical trial de-risking, and hitting enrollment targets on schedule removes execution uncertainty.
What Changed From Last Quarter?
*Estimated based on burn rate
The key delta is the D2T RA enrollment completion — this was the most anticipated near-term catalyst and positions the company for pivotal data readouts later this year. The December financing diluted shareholders by ~19% but provided runway security through commercialization.
What Did Management Say in the Q&A?
The earnings call Q&A provided important color on competitive positioning, data expectations, and commercial strategy:
On Argenx Competition in Graves' Disease
"We have a lead in Graves that will be significant roughly no matter what design Argenx runs. We have great relationships with those KOLs and doc community... One of our studies is also 26 weeks."
Management emphasized IMVT-1402's differentiation through deeper IgG suppression:
"We showed pretty conclusively in our phase 2 data that the deeper IgG suppression that we expect to deliver will matter in this population, especially on remission."
On D2T RA Data Expectations
Management acknowledged the high unmet need but noted limited precedent data for drugs in late-stage RA with this level of pretreatment. They plan to provide guidance on "what would cause us to run the second study" before releasing data.
On MG Launch Timing
While the catalyst slide showed Graves' launch by end of 2028 but not MG:
"There's probably some possibility [MG] actually does, in fact, also launch in 2028."
On Batoclimab TED Read-Through
Management downplayed read-through from TED to Graves' disease:
"We don't think there's a lot of read-through from TED... The diseases are pretty different. The TED study enrolled mostly euthyroid patients... I feel like we are confident in the efficacy or potential efficacy of FCRNs in Graves' disease and not particularly focused on what information there is from TED."
What Are the Key Risks?
The 8-K highlighted several risk factors:
- Clinical Execution Risk — Multiple registrational trials running simultaneously requires flawless execution
- Regulatory Uncertainty — FDA approval timelines and requirements could shift
- Competitive Landscape — Anti-FcRn is a competitive space with argenx (ARGX) already commercializing Vyvgart
- Capital Requirements — Despite strong cash position, commercialization will require significant investment
- Macro/Supply Chain — International trade tariffs and geopolitical factors could impact operations
What Catalysts Are Ahead?
The next major catalyst is the TED Phase 3 readout in H1 2026, which will inform the market about batoclimab's viability. Management indicated both TED studies will report in H1 2026.
The bigger focus for investors is IMVT-1402, which has differentiated dosing (subQ autoinjector) and a broader indication pipeline. Management expects to report both the open-label and randomized withdrawal data from D2T RA together in H2 2026.
Bottom Line
Immunovant delivered a clean beat on EPS while hitting the critical D2T RA enrollment milestone. With nearly $1B in cash extending runway to Graves' disease commercialization, the focus now shifts to execution on multiple data readouts in 2026-2027. The +7% aftermarket reaction signals investor confidence in the pipeline trajectory.
Key Numbers:
- EPS: -$0.61 vs -$0.73 expected (beat by 10.6%)
- Cash: $994.5M (runway to GD launch)
- Stock: +7.4% aftermarket
Read the full Q3 2026 earnings transcript.
Updated February 6, 2026 with earnings call transcript highlights.