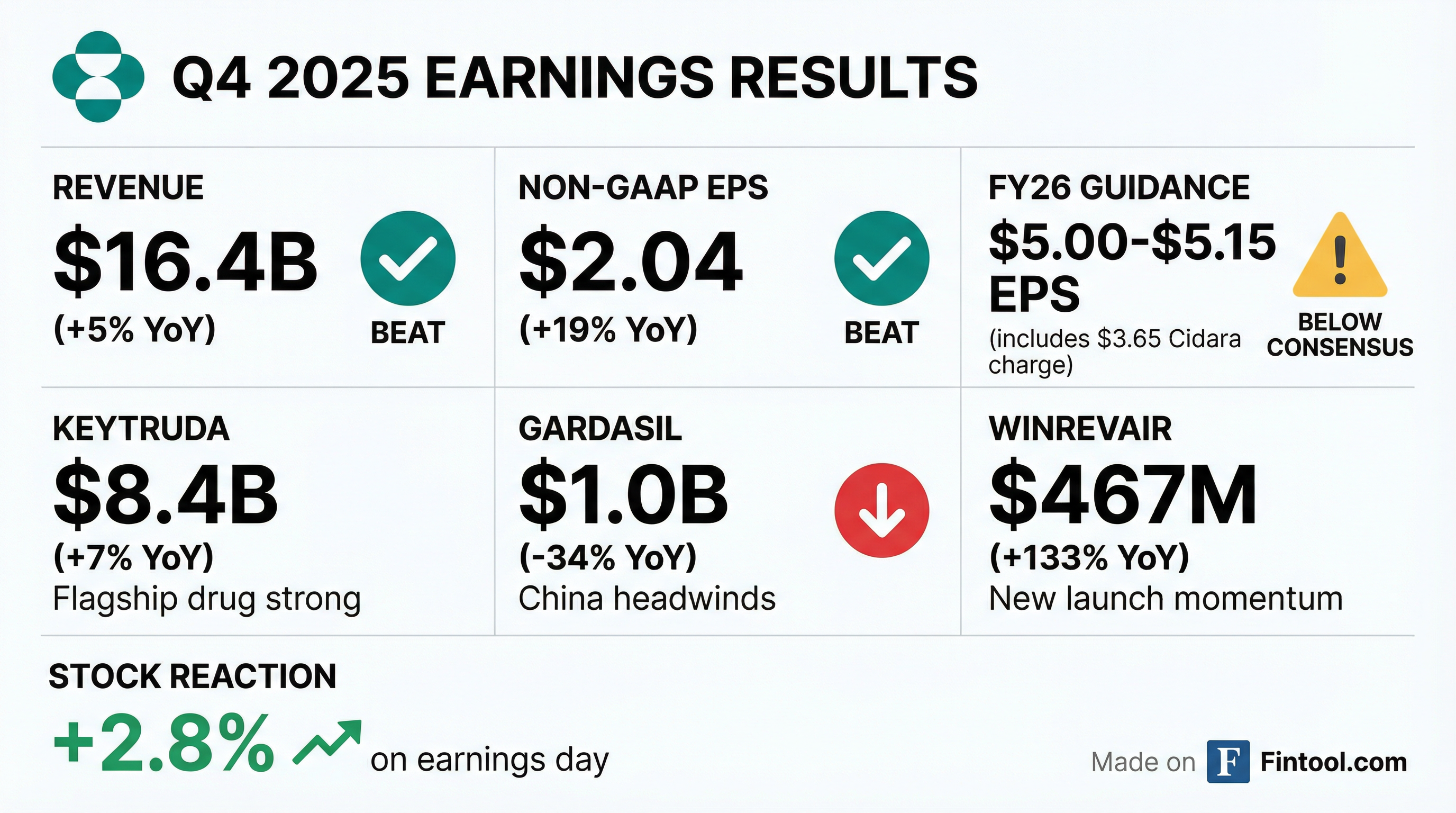
Earnings summaries and quarterly performance for Merck & Co..
Executive leadership at Merck & Co..
Robert M. Davis
Chief Executive Officer and President
Betty Larson
Executive Vice President and Chief Human Resources Officer
Caroline Litchfield
Executive Vice President and Chief Financial Officer
Dean Li
Executive Vice President and President, Merck Research Laboratories
Richard R. DeLuca, Jr.
Executive Vice President and President, Merck Animal Health
Board of directors at Merck & Co..
Christine E. Seidman, M.D.
Director
Douglas M. Baker, Jr.
Director
Inge G. Thulin
Director
Kathy J. Warden
Director
Mary Ellen Coe
Director
Pamela J. Craig
Director
Patricia F. Russo
Director
Paul B. Rothman, M.D.
Director
Risa J. Lavizzo-Mourey, M.D.
Director
Stephen L. Mayo, Ph.D.
Director
Surendralal L. Karsanbhai
Director
Thomas H. Glocer
Lead Independent Director
Research analysts who have asked questions during Merck & Co. earnings calls.
Mohit Bansal
Wells Fargo & Company
7 questions for MRK
Umer Raffat
Evercore ISI
7 questions for MRK
Akash Tewari
Jefferies
6 questions for MRK
Daina Graybosch
Leerink Partners
6 questions for MRK
James Shin
Analyst
6 questions for MRK
Christopher Schott
JPMorgan Chase & Co.
5 questions for MRK
Vamil Divan
Guggenheim Securities
5 questions for MRK
Evan Seigerman
BMO Capital Markets
4 questions for MRK
Steve Scala
Cowen
4 questions for MRK
Terence Flynn
Morgan Stanley
4 questions for MRK
Trung Huynh
UBS Group AG
4 questions for MRK
Alexandria Hammond
Wolfe Research
3 questions for MRK
Carter L. Gould
Barclays
3 questions for MRK
Courtney Breen
AllianceBernstein
3 questions for MRK
Alex Hammond
Sidoti & Company, LLC
2 questions for MRK
Asad Haider
Goldman Sachs
2 questions for MRK
Chris Schott
JPMorgan Chase & Company
2 questions for MRK
Geoff Meacham
Citigroup Inc.
2 questions for MRK
Geoffrey Meacham
Citi
2 questions for MRK
Luisa Hector
Berenberg
2 questions for MRK
Tim Anderson
Bank of America
2 questions for MRK
Timothy Anderson
BofA Securities
2 questions for MRK
Chris Shibutani
Goldman Sachs Group, Inc.
1 question for MRK
Louise Chen
Cantor Fitzgerald
1 question for MRK
Recent press releases and 8-K filings for MRK.
- FDA approves NUMELVI (atinvicitinib), the first second-generation JAK inhibitor for control of pruritus in dogs six months and older; availability expected in spring 2026.
- Once-daily treatment with high JAK1 selectivity (>10× vs JAK2, JAK3, TYK2), offering a favorable safety profile and reducing itch from the first dose.
- Designed for convenience: treats dogs weighing as little as 4.4 lb, requires no vaccination schedule adjustments, and features a stable formulation with extended shelf life.
- Addresses a significant market need, as skin conditions account for up to 20 % of cases in general veterinary practice and negatively impact animal and owner quality of life.
- Doravirine/islatravir (DOR/ISL), Merck’s once-daily two-drug regimen, met primary endpoints in three Phase 3 trials with non-inferior efficacy and a comparable safety profile in both treatment-naïve and switch patients.
- In the MK-8591A-053 trial, DOR/ISL achieved 91.8% viral suppression at week 48 versus 90.6% for the comparator BIC/FTC/TAF.
- Two additional trials (MK-8591A-052 and MK-8591A-051) showed DOR/ISL maintained durable viral suppression through week 96.
- The Phase 3 data support Merck’s New Drug Application for DOR/ISL, with an FDA target action date of April 28, 2026.
- Doravirine is already approved in the U.S. for HIV-1 treatment, providing an established safety profile for the combination.
- Merck presented Phase 3 results for investigational doravirine/islatravir (DOR/ISL) showing non-inferior viral suppression at Week 48 versus BIC/FTC/TAF (91.8% vs. 90.6%; treatment difference 1.2%) in treatment-naïve adults, with similar safety profiles (drug-related AEs 14% vs. 18%; discontinuations 1.1% vs. 2.2%).
- DOR/ISL maintained high rates of virologic suppression through Week 96 in both treatment-naïve and switch trials, with no new safety signals and comparable discontinuation rates.
- The U.S. FDA has set a PDUFA target action date of April 28, 2026 for the DOR/ISL New Drug Application, potentially expanding Merck’s HIV treatment portfolio.
- Second-season Phase 3 SMART trial data show ENFLONSIA (clesrovimab) was well tolerated in children under 2 at increased risk for severe RSV, with safety profiles consistent with season 1 and no drug-related serious adverse events reported.
- Pharmacokinetic exposures in season 2 participants matched levels seen in healthy infants from the pivotal CLEVER trial, supporting efficacy extrapolation for the second RSV season.
- Through Day 180 (6 months) in season 2, RSV-associated medically attended lower respiratory infection and hospitalization rates were 7.3% and 3.0%, respectively, reflecting higher baseline risk post-pandemic.
- Merck plans to submit these findings to the FDA and other regulators to seek an expanded indication for ENFLONSIA in children entering their second RSV season; the antibody is already approved for first-season use in the U.S., Canada and select markets.
- Merck and Mayo Clinic launch a research and development collaboration to apply AI and advanced analytics in drug discovery and precision medicine.
- Merck will integrate Mayo Clinic Platform’s de-identified clinical and genomic datasets, advanced AI tools and analytics to improve target identification and early development decisions.
- The collaboration initially targets high-need areas: IBD, atopic dermatitis, and multiple sclerosis.
- This represents Mayo Clinic’s first large-scale strategic collaboration with a global biopharmaceutical company.
- Poxel generated consolidated revenue of €5.0 million in FY 2025, down from €6.6 million in FY 2024
- Fourth-quarter TWYMEEG® gross sales in Japan rose 15% sequentially and 40% year-on-year, with royalties at 10% in Q1/Q4 and 8% in Q2/Q3 (including retroactive adjustments)
- Cash and cash equivalents totaled €0.9 million as of December 31, 2025, up from €0.6 million at September 30, 2025
- Lyon Commercial Court approved Poxel’s continuation plan on January 22, 2026, ending its judicial reorganisation proceedings
- Poxel expects double-digit royalty growth on TWYMEEG® sales in 2026 under its recovery plan
- Chinese prosecutors have charged an AstraZeneca subsidiary and former China head Leon Wang with unlawful data collection, illegal trade and medical insurance fraud, consolidating November 2025 indictments into a single proceeding.
- Authorities allege about 24 million yuan (≈$3.5 million) in unpaid import taxes on cancer drugs Imfinzi, Imjudo and Enhertu, which AstraZeneca contends it prepaid.
- China accounted for about 11% of AstraZeneca’s $58.7 billion revenue in 2025, underscoring the market’s materiality amid legal uncertainty.
- Following the indictments, AstraZeneca has overhauled its China leadership, appointing a new international executive vice president, and reaffirmed a $15 billion investment pledge in the region.
- Helus Pharma appoints Michael Cola as CEO to lead its next phase of CNS-focused R&D and commercialization executing key pipeline milestones.
- Cola brings over 30 years of experience in neuroscience, rare diseases and specialty pharmaceuticals, including senior roles at Shire, Astra-Merck, AstraZeneca and Avalo Therapeutics.
- Under his leadership, Helus will advance key clinical programs—processing upcoming HLP004 II data and planning for HLP003 III topline results in Q4 2026—while strengthening its global 350+ patent portfolio.
- Helus Pharma appoints Michael Cola as CEO, effective immediately.
- Cola brings 30+ years of experience in neuroscience and specialized pharmaceuticals, including leadership at Shire’s Specialty Pharmaceutical division, Astra-Merck and AstraZeneca.
- He led Shire’s market cap growth from $5 billion to $20 billion and the expansion of Vyvanse into a multibillion-dollar franchise.
- Helus expects Phase 2 HLP004 data this quarter and Phase 3 HLP003 top-line data in Q4 2026, while focusing on global regulatory engagement and long-term commercial planning.
- The company holds a robust IP portfolio with 350+ patent applications pending and 100+ patents granted worldwide.
- Microbiotica’s oral precision microbiome therapy MB310 met primary and secondary endpoints in 29 ulcerative colitis patients, showing a safety profile comparable to placebo and successful engraftment of all eight bacterial strains.
- In the intention-to-treat analysis, 63.2% (12/19) of MB310-treated patients achieved clinical remission versus 30.0% (3/10) on placebo, with all responders remaining in remission and resolving rectal bleeding during follow-up.
- The trial demonstrated improvements in histological markers of mucosal damage and reductions in inflammatory biomarkers, including fecal calprotectin, suggesting a potential disease-modifying mechanism via gut barrier restoration and immune regulation.
- Microbiotica plans an adaptive Phase 2/3 program combining MB310 with standard anti-inflammatory or immunomodulatory agents and is evaluating partnering and financing options for late-stage development and commercialization.
- Investors IP Group and Flerie hold undiluted stakes of approximately 16.7–17% (valued at ~£13.9 million) and 10%, respectively, underscoring strong backing if larger trials confirm these findings.
Fintool News
In-depth analysis and coverage of Merck & Co..

Merck Splits Pharma Division as $30B Keytruda Cliff Looms

Merck Beats Q4 Estimates But Guides Low as Gardasil Headwinds Persist

Merck Walks Away From $30 Billion Revolution Medicines Deal; Stock Plunges 22%

Merck Emerges as Lead Suitor for Revolution Medicines at $28-32 Billion

Patient Deaths Halt Merck's $5.5 Billion ADC Trial: Second Major Blow to Daiichi Partnership
Quarterly earnings call transcripts for Merck & Co..
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more