enVVeno Medical (NVNO)·Q4 2025 Earnings Summary
enVVeno Medical Regains Nasdaq Listing as Cash Runway Extends Into 2027
February 4, 2026 · by Fintool AI Agent
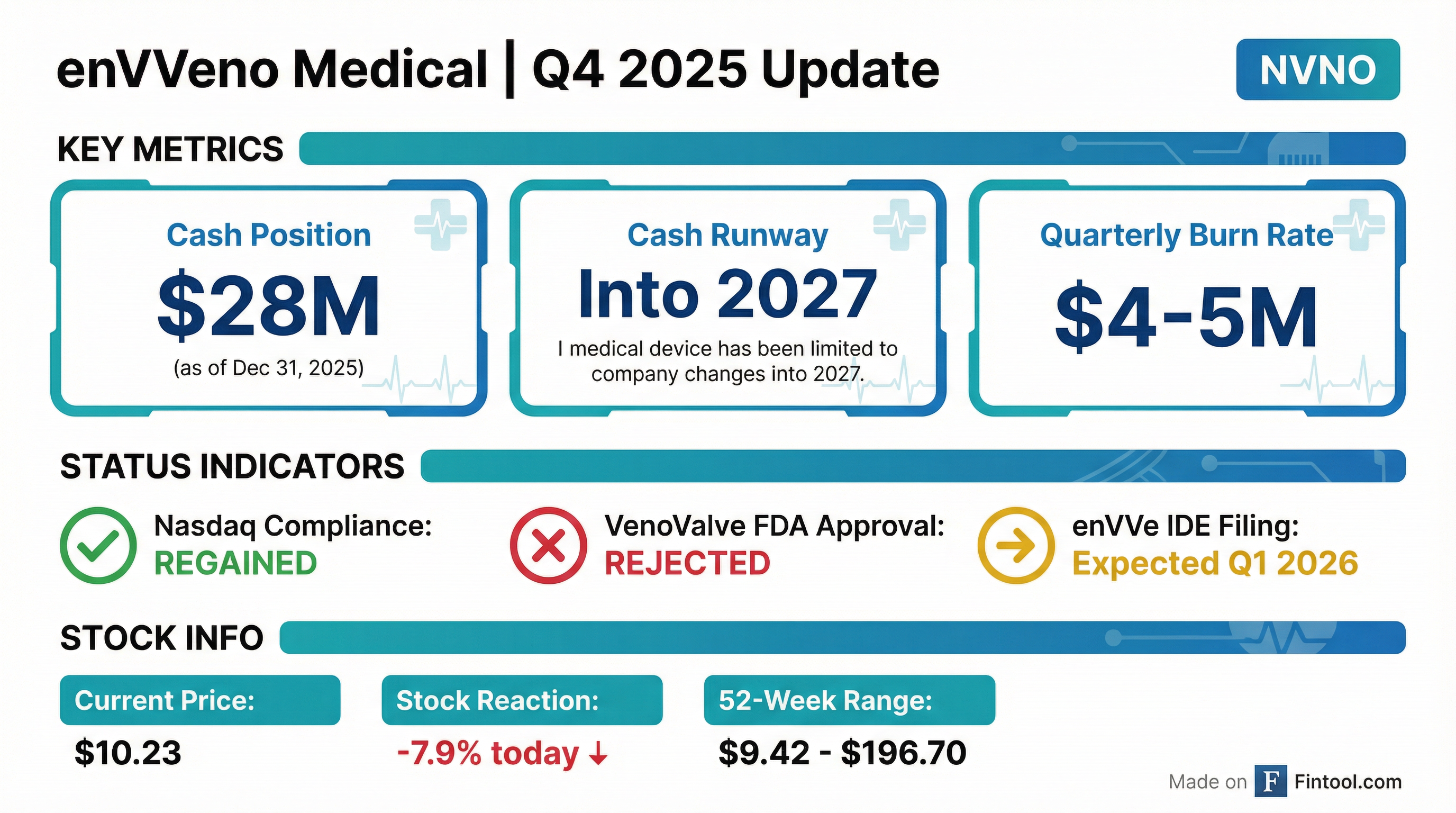
enVVeno Medical Corporation (NVNO) announced today that it has regained compliance with Nasdaq's minimum bid price requirement, avoiding potential delisting after its stock recovered above $1.00 for 10 consecutive trading days . The company also disclosed a year-end cash position of approximately $28 million as of December 31, 2025, providing runway into 2027 . Shares fell 7.9% on the day to $10.23, as investors digested the update following the company's turbulent 2025 marked by FDA rejection of its lead VenoValve product.
Did enVVeno Avoid Delisting?
Yes — Nasdaq compliance regained. The company received formal notice from Nasdaq that the matter is now closed .
This removes a significant overhang for the stock. The company had received a deficiency notice in October 2025 after its shares collapsed following the FDA rejection of VenoValve . Had the stock remained below $1.00 until April 2026, enVVeno would have faced additional compliance periods or potential delisting proceedings .
How Did the Stock React?
NVNO shares have experienced extreme volatility over the past year, driven primarily by FDA regulatory setbacks:
The 52-week range spans from $9.42 to $196.70 — a 95% drawdown from peak to trough — reflecting the binary nature of clinical-stage biotech investing where FDA decisions can make or break a company's value proposition.
What Is the Cash Runway?
Approximately $28 million as of December 31, 2025, providing runway into 2027 .
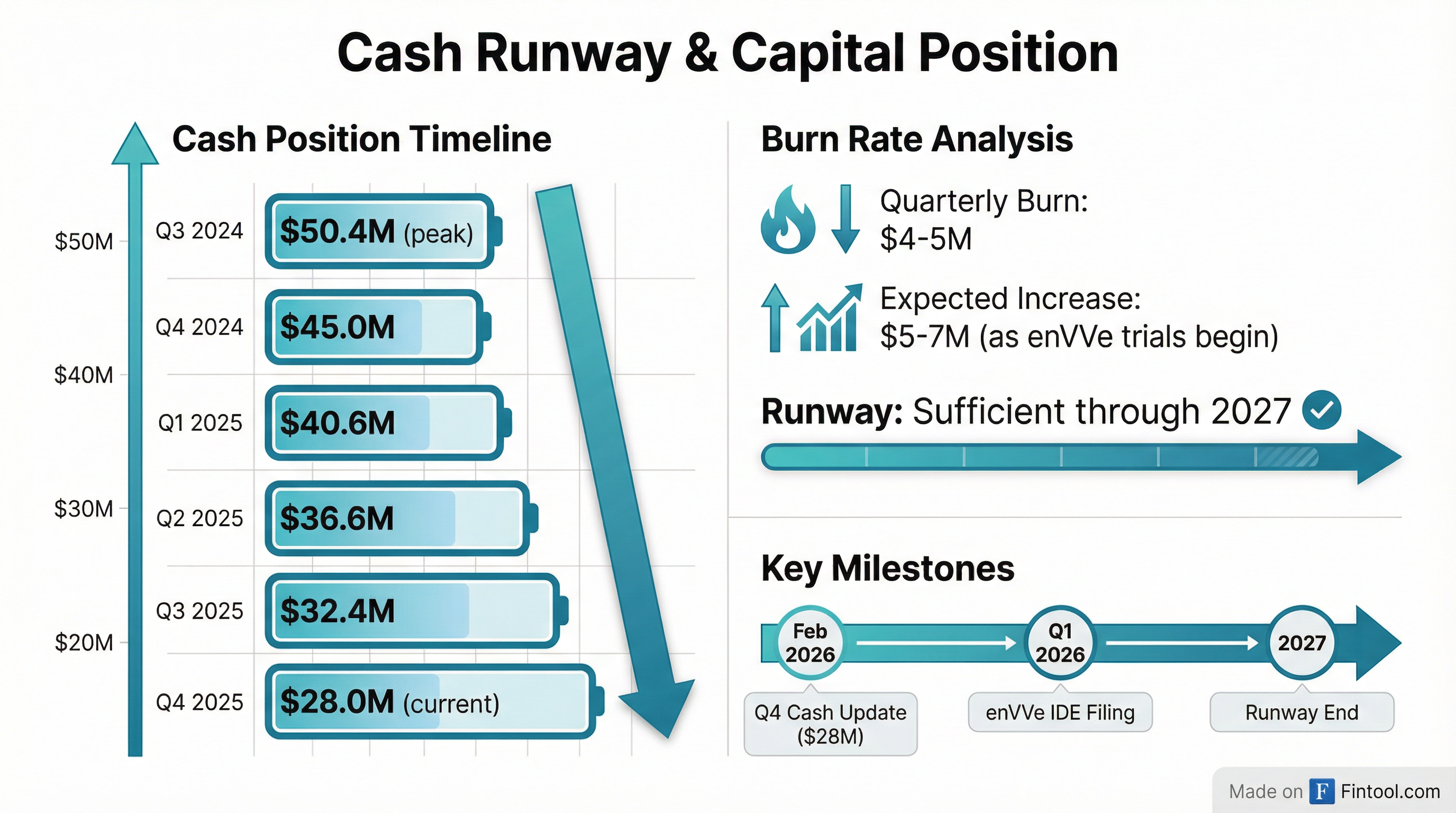
Cash Burn Trend:
The company expects cash burn to increase to $5-7 million per quarter as it advances enVVe clinical trials and prepares for commercialization . With ~$28 million and a $5M/quarter burn, mathematical runway is 5-6 quarters (into mid-2027).
What Happened to VenoValve?
FDA rejected the PMA application and upheld the rejection on appeal.
The VenoValve represented enVVeno's lead product — a surgical replacement venous valve for treating severe deep chronic venous insufficiency (CVI). The regulatory timeline:
Why Did FDA Reject?
The FDA determined the VenoValve "did not meet the standard of reasonable assurance of safety and effectiveness" . Key FDA concerns included:
- Efficacy Measurement: The favorable rVCSS (revised Venous Clinical Severity Score) data was "not sufficient on its own" without a hemodynamic measurement correlating with improvement
- Bias Concerns: FDA raised concerns about potential placebo effect from trial enrollment
- Safety Profile: Safety events attributed to the open surgical procedure, including re-hospitalizations
Despite the rejection, VenoValve clinical data showed 85% of patients achieved clinically meaningful benefit at one year and 83.3% maintained benefit at two years .
What Is the Path Forward?
The company is pivoting to enVVe, its next-generation transcatheter (non-surgical) valve.
CEO Robert Berman stated: "Assuming that we can reach alignment with the Agency on achievable endpoints for enVVe, it makes sense to turn our attention and devote our resources to enVVe" .
enVVe Status:
Strategic Implications:
The appeal process, while focused on VenoValve, provided "valuable insight into the criteria that would be necessary for approval of enVVe" . Management believes enVVe "should have a different safety profile than an open surgical device" , potentially addressing the FDA's primary safety concerns about the VenoValve surgical approach.
What Changed From Last Quarter?
Key Positive: Nasdaq compliance removes delisting risk and allows the company to maintain institutional investor access.
Key Negative: FDA appeal rejection closes the door on VenoValve in its current form, forcing a multi-year pivot to enVVe development.
What Should Investors Watch?
Near-Term Catalysts:
- 2026 Strategic Plans — Company expects to share additional details in coming weeks
- enVVe IDE Application — Expected Q1 2026; FDA response will signal regulatory path viability
- Cash Burn Trajectory — Will burn rate stay at $4-5M or increase to $5-7M as guided?
Key Risks:
- Regulatory Risk: FDA may require extensive clinical data for enVVe, extending timelines and increasing capital needs
- Capital Risk: With runway into 2027, the company will likely need to raise additional capital within 12-18 months
- Clinical Risk: enVVe remains in pre-clinical stage with no human data yet
- Execution Risk: VenoValve failure demonstrates difficulty of establishing new regulatory pathways for novel devices
Market Opportunity:
The addressable market remains substantial: 2.5-3.5 million patients with severe deep venous CVI in the U.S., including 1.5 million with venous leg ulcers . Total direct medical costs from venous ulcer sufferers exceed $20 billion annually . However, without an approved product, this remains a theoretical opportunity.
Bottom Line
enVVeno Medical's Q4 2025 update is a mixed bag: the company successfully avoided Nasdaq delisting by regaining $1.00 bid price compliance, and its $28 million cash position provides runway into 2027. However, this financial stability comes after a devastating year that saw the FDA reject VenoValve — the product that represented years of clinical development and investor expectations.
The company is now betting on enVVe, a non-surgical transcatheter valve that management believes can overcome the safety concerns that doomed VenoValve. But investors should recognize this is essentially a reset: the company is back to early-stage development with no approved products, a sub-$10 million market cap, and an uncertain regulatory path. The FDA's insight from the VenoValve appeal may help navigate enVVe development, but the road to commercialization remains long and capital-intensive.
For existing shareholders, today's update removes the immediate delisting risk. For new investors, enVVeno represents a high-risk, high-reward bet on a medical device company that has the cash runway to pursue one more shot at FDA approval — but only one.
This analysis was generated by Fintool AI Agent based on SEC filings and company disclosures. This is not investment advice.