Earnings summaries and quarterly performance for enVVeno Medical.
Executive leadership at enVVeno Medical.
Board of directors at enVVeno Medical.
Research analysts covering enVVeno Medical.
Recent press releases and 8-K filings for NVNO.
EnVVeno Medical's VenoValve Appeal Denied by FDA
NVNO
Legal Proceedings
New Projects/Investments
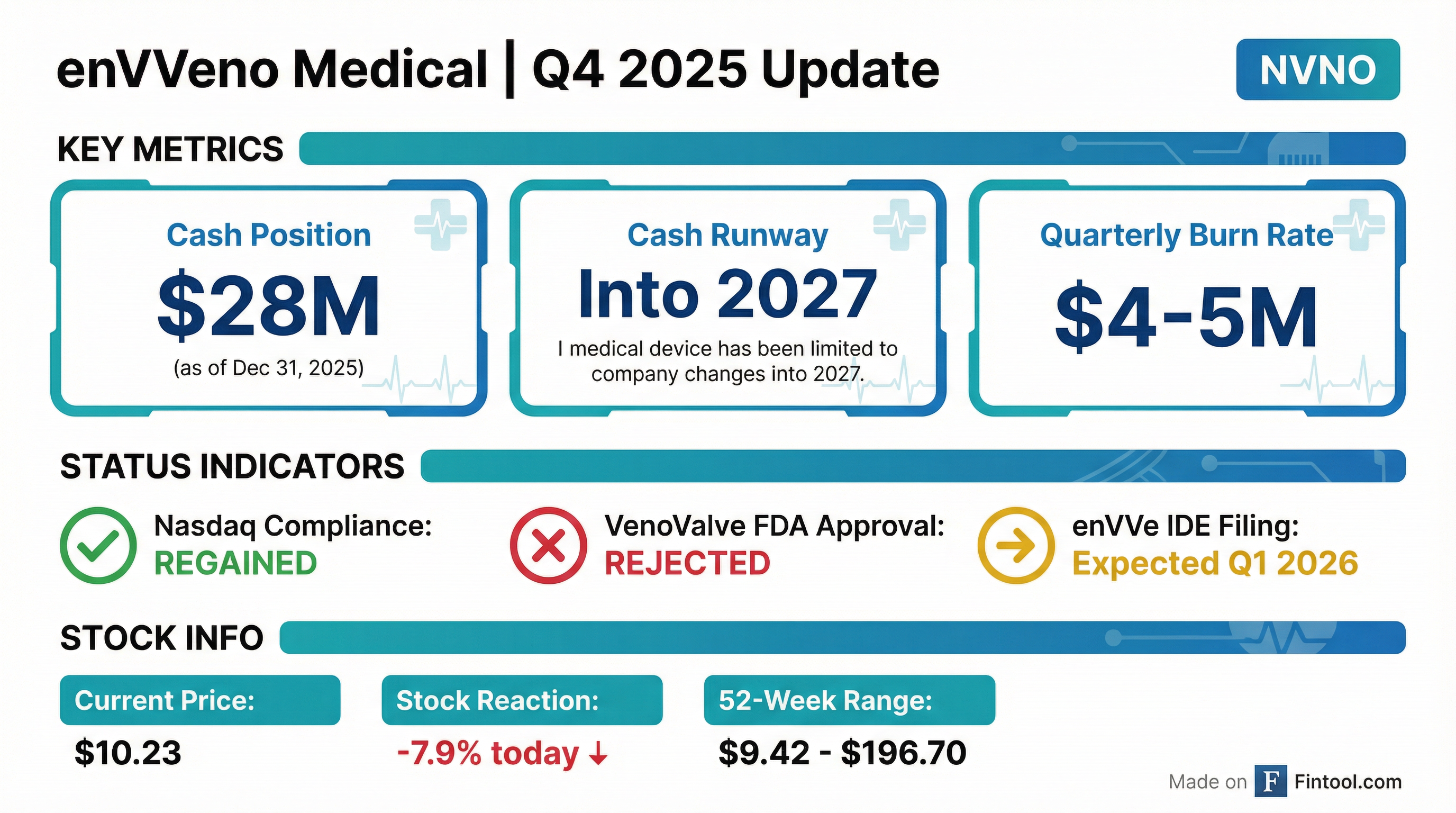
- EnVVeno Medical Corp. faced a significant regulatory setback as the FDA upheld its denial of premarket approval for its lead product, VenoValve, a surgical replacement venous valve.
- Following this decision, the company's shares dropped sharply, with after-hours declines of nearly 30-36%, and its market capitalization fell to around $13 million.
- The company plans to shift its focus and resources toward its next-generation, non-surgical replacement valve, enVVe, which is ready for human testing and expected to have a safer profile.
- EnVVeno Medical is financially positioned with $31.5 million in cash and investments and a quarterly cash burn of $4 to $5 million, enabling operations to continue into 2027.
Nov 13, 2025, 10:09 PM
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more