NOVARTIS (NVS)·Q4 2025 Earnings Summary
Novartis Hits 40% Margin Target Two Years Early, Stock Jumps 3%
February 4, 2026 · by Fintool AI Agent
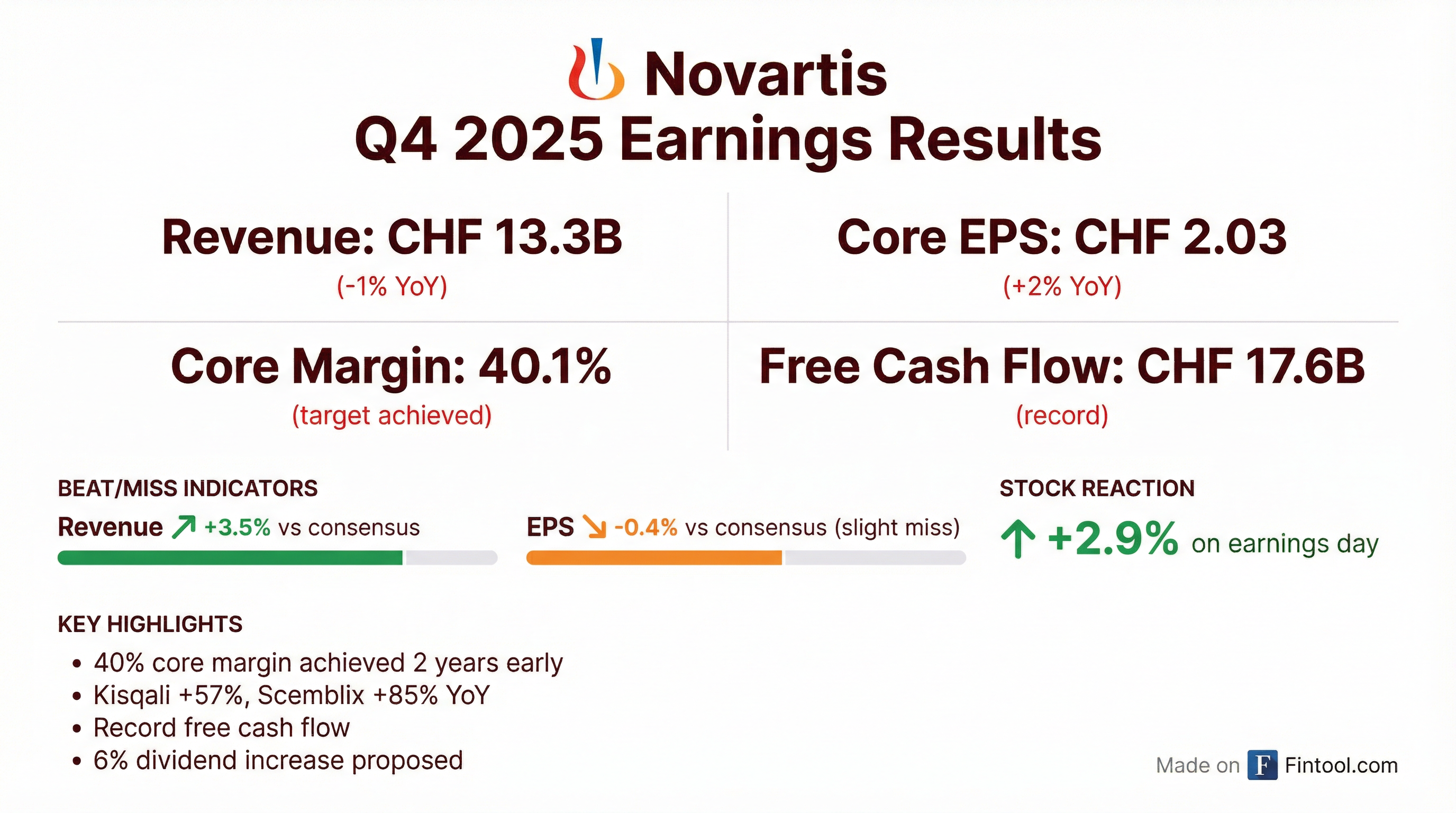
Novartis delivered mixed Q4 2025 results with revenue beating consensus by 3.5% while EPS slightly missed by 0.4%, but the headline story is the company achieving its 40% core margin target two years ahead of schedule . The stock rallied 2.9% on the news as investors focused on the operational execution and record CHF 17.6B free cash flow .
Did Novartis Beat Earnings?
Novartis posted Q4 2025 revenue of CHF 13.3B, beating the CHF 12.9B consensus by 3.5% . However, Q4 sales declined 1% YoY due to the Entresto loss of exclusivity and gross-to-net adjustments impacting Kisqali .
Core EPS came in at CHF 2.03, essentially in line with estimates (slight miss of 0.4%) . For the full year, Novartis delivered 8% sales growth, 14% core operating income growth, and 17% core EPS growth to CHF 8.98 .
*Values retrieved from S&P Global
How Did the Stock React?
NVS shares jumped 2.9% on earnings day, rising from $149.86 to $154.24 — hitting a new 52-week high . The positive reaction reflects investor confidence in the company's ability to grow through the largest patent expiry in its history.
Key factors driving the stock reaction:
- 40% margin achievement two years ahead of 2027 target
- Record free cash flow of CHF 17.6B, up 8% YoY
- 6% dividend increase proposed — the 29th consecutive increase since company creation
- Strong priority brand performance offsetting Entresto generics
What Are the Key Growth Drivers?
Novartis's priority brands delivered 35% combined growth, demonstrating the company's ability to navigate through patent cliffs .
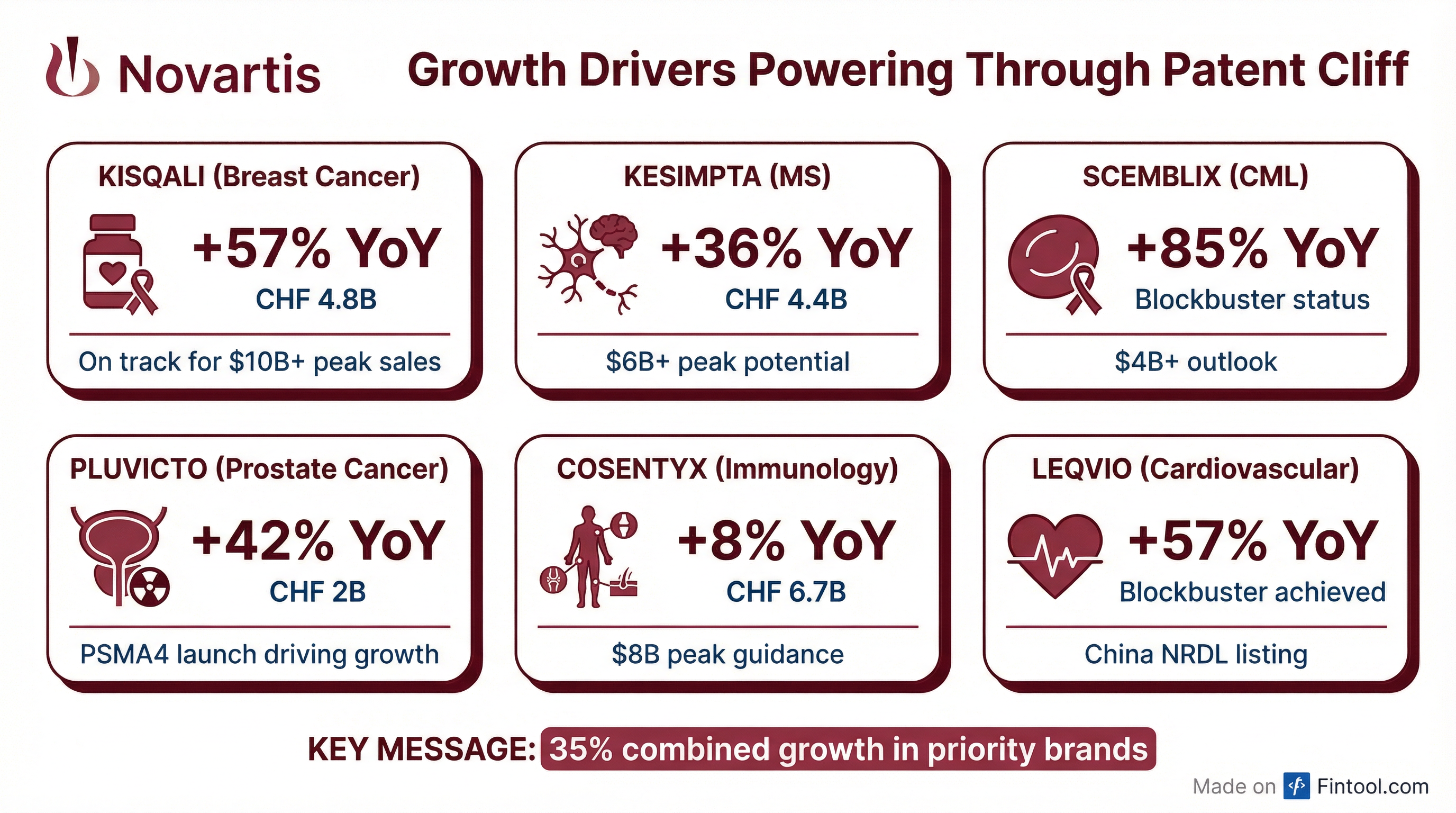
Brand-by-Brand Performance
Kisqali is on track to become the largest brand in Novartis history, with US EBC NBRX share above 60% and Germany EBC share over 80% .
Pluvicto reached $2B in sales with 75% US growth driven by PSMA4 launch. The company has secured approvals in Japan and China, with hormone-sensitive prostate cancer SNDA submitted to FDA .
Rhapsido (remibrutinib) just launched in CSU with encouraging early results — over 2,000 HCP starts in sampling/bridge programs, benchmarking well versus successful dermatology launches .
What Did Management Guide?
For 2026, Novartis expects:
- Sales: Low single-digit growth
- Core Operating Income: Low single-digit decline
- Core Margin Dilution: 1-2 percentage points from Avidity deal
This is a "year of two halves" — H1 will see sales decline low single-digit and core operating income decline low double-digit due to tough Entresto/Promacta/Tasigna comps. H2 expects sales up mid-single-digit and core operating income up mid-to-high single-digit .
Long-term, management reaffirmed:
- 5-6% sales CAGR through 2030
- Return to 40%+ core margin by 2029
- Mid-single-digit sales growth expected in the 2030s
What Changed From Last Quarter?
Margin Milestone: The 40% core margin achievement was the biggest change — originally targeted for 2027, now delivered two years early .
CFO Transition: Harry Kirsch is retiring after 13 years as CFO. Mukul Mehta takes over in mid-March. Kirsch was credited with the company's financial discipline and productivity culture .
Pipeline Developments:
- Remibrutinib submitted for CIndU (chronic inducible urticaria)
- Pelabresib now has EU and US path forward after positive 96-week data
- Pelacarsen CVRR readout expected mid-2026
- Zigakibart IgAN readout pushed to H1 2027 to align UPCR and eGFR data
Strategic Shift: Management acknowledged MFN (Most Favored Nation) pricing impact on ex-US launches, particularly for Ianalumab. They're working through pricing strategies but expressed confidence in Rhapsido's global launch path .
Key Q&A Highlights
On Remibrutinib in MS: CEO Vas Narasimhan expressed confidence in the drug's liver safety profile, noting no Hy's Law cases and an already approved CSU label without liver warnings. FDA requested limited liver monitoring "out of an abundance of caution," but Novartis plans to advocate for maintaining the clean label if phase 3 trials remain signal-free .
On Pelacarsen Delay: The Horizon trial readout slipped to mid-2026 due to lower-than-expected event rates. Management believes this reflects optimal patient management on other risk factors (especially LDL lowering) rather than issues with trial design. The study remains powered for 20% CVRR at 70mg/dL and 25% at 90mg/dL .
On Oral SERDs Competition: Management is not concerned about oral SERDs threatening Kisqali in early breast cancer. They expect CDK4/6 inhibitors to remain standard of care, with oral SERDs potentially becoming the preferred endocrine therapy partner .
On Food Allergy Opportunity: Positive phase 2 data for remibrutinib in food allergy has shifted management's perspective to viewing this as a "multi-billion dollar opportunity." Phase 3 program now initiating .
What's the Pipeline Outlook?
Novartis has 7 pivotal readouts expected in 2026 :
The Avidity Biosciences acquisition is expected to close H1 2026, bringing AOC 1001 (DMD) and AOC 1044 (DM1) into the pipeline .
Capital Allocation & Shareholder Returns
Novartis maintained its balanced capital allocation strategy:
- R&D Investment: $10B+ in 2025, up 8% YoY
- M&A: 4 acquisitions and 10 licensing deals in 2025
- Share Buybacks: CHF 7.7B remaining on up-to-CHF 10B program (through 2027)
- Dividend: CHF 3.70/share proposed (+6%), 29th consecutive increase
Bottom Line
Novartis delivered on operational execution in Q4 2025, achieving its 40% core margin target two years early despite the largest patent expiry in company history. The priority brand portfolio grew 35% and provides clear visibility to the 5-6% CAGR guidance through 2030. While 2026 will be a transition year (particularly H1), the setup into H2 and beyond looks increasingly favorable. The 2.9% stock pop reflects growing confidence that Novartis can grow through the Entresto cliff.
Key Risks to Monitor:
- Pelacarsen trial outcome (mid-2026) — binary event for CV franchise
- Avidity integration execution
- MFN pricing impact on ex-US launches
- Kisqali patent expiry timing (mid-2031 with pediatric extension)