Earnings summaries and quarterly performance for NOVARTIS.
Research analysts who have asked questions during NOVARTIS earnings calls.
James Quigley
Goldman Sachs
8 questions for NVS
Peter Verdult
Citigroup Inc.
7 questions for NVS
Simon Baker
Redburn Atlantic
7 questions for NVS
Florent Cespedes
Bernstein
6 questions for NVS
Matthew Weston
UBS Group AG
6 questions for NVS
Richard Vosser
JPMorgan Chase & Co.
6 questions for NVS
Seamus Fernandez
Guggenheim Partners
6 questions for NVS
Thibault Boutherin
Morgan Stanley
6 questions for NVS
Graham Parry
Bank of America Corporation
5 questions for NVS
Rajesh Kumar
HSBC
4 questions for NVS
Emmanuel Papadakis
Deutsche Bank
3 questions for NVS
Kerry Holford
Berenberg
3 questions for NVS
Michael Leuchten
Jefferies
3 questions for NVS
Sachin Jain
Bank of America
3 questions for NVS
Steve Scala
Cowen
3 questions for NVS
Emily Field
Barclays
2 questions for NVS
James Gordon
JPMorgan Chase & Co.
2 questions for NVS
Michael Lyson
Jefferies Financial Group Inc.
2 questions for NVS
Richard Foster
JPMorgan Chase & Co.
2 questions for NVS
Richard Parkes
BNP Paribas Exane
2 questions for NVS
Sachin Dean
Bank of America Corporation
2 questions for NVS
Shirley Tan
Barclays PLC
2 questions for NVS
Steven Scala
TD Cowen
2 questions for NVS
Steven Skiena
The Toronto-Dominion Bank
2 questions for NVS
Eric Le Berrigaud
Stifel
1 question for NVS
Etzer Darout
BMO Capital Markets
1 question for NVS
Graham Parry
Bank of America
1 question for NVS
Harry Sephton
UBS
1 question for NVS
Jo Walton
UBS
1 question for NVS
Mark Purcell
Morgan Stanley
1 question for NVS
Michael Nedelcovych
TD Cowen
1 question for NVS
Naresh Chouhan
Intrinsic Health
1 question for NVS
Peter Welford
Jefferies
1 question for NVS
Shirley Chen
Barclays
1 question for NVS
Timothy Anderson
BofA Securities
1 question for NVS
Recent press releases and 8-K filings for NVS.
- Novartis reiterated a prior commitment to invest approximately $23 billion to build or expand U.S. operations, including recent groundbreakings for manufacturing and research sites in North Carolina and California, and plans for a radioligand therapy manufacturing site in Florida.
- President Donald Trump claimed his tariff policies spurred this expansion and that Novartis would build 11 new U.S. facilities; however, Novartis's statement did not validate this specific figure or explicitly link the expansions to the administration's tariff actions, having previously announced plans for 10 U.S. facilities.
- Markets and analysts are awaiting clearer operational details before treating the announcement as a concrete shift in corporate capital allocation.
- Novartis is a significant U.S. market participant, generating roughly one-third of its global revenue in the United States and possessing robust financial strength metrics.
- Private equity firm ChrysCapital has agreed to acquire a 70.68% stake in Novartis India Ltd from Novartis AG for approximately Rs 1,446 crore.
- The acquisition includes a mandatory open offer at Rs 860.64 per share, which could increase ChrysCapital's total holding to over 96% if successful.
- Following the deal, Novartis AG will divest its entire stake in Novartis India, and the Novartis brand is expected to be phased out from the listed entity. Novartis AG will continue to operate in India through its wholly owned Novartis Healthcare Pvt Ltd.
- The global market for radioligand therapeutics in cancer treatment is projected to expand significantly, from an estimated $2.6 billion in 2025 to $4.8 billion by 2030, representing a Compound Annual Growth Rate (CAGR) of 13.1%.
- Novartis AG's Pluvicto and Lutathera have played a crucial role in catalyzing commercial momentum within the approved radioligand therapy market.
- The market is expected to see increased adoption across North America, Europe, and emerging markets, with Novartis AG anchoring the North American market through substantial sales.
- Novartis has signed a research collaboration and licensing agreement with Unnatural Products to develop orally delivered macrocyclic peptide therapeutics for cardiovascular disease.
- The deal is valued at up to $1.8 billion, with Unnatural Products eligible for up to $100 million in upfront and pre-IND milestone payments, and potentially up to $1.7 billion in development, regulatory, and commercial milestones.
- Novartis will assume responsibility for IND-enabling studies, clinical development, manufacturing, and global commercialization of the therapies.
- The partnership combines Unnatural Products' AI-enhanced macrocycle discovery engine with Novartis's global development and commercialization capabilities to pursue next-generation cardiovascular therapies.
- Novartis announced positive Phase 3 ALIGN results for Vanrafia (atrasentan) in IgA nephropathy, demonstrating a clinically meaningful slowing of kidney function decline.
- Despite narrowly missing a key statistical endpoint in the ALIGN trial, Novartis intends to pursue traditional FDA approval for Vanrafia, leveraging regulatory precedent for similar IgAN drugs.
- Vanrafia, a potent endothelin A (ETA) receptor antagonist, was acquired by Novartis in June 2023 as part of its £2.5 billion purchase of Chinook Therapeutics and received accelerated approvals in the U.S. and China in 2025.
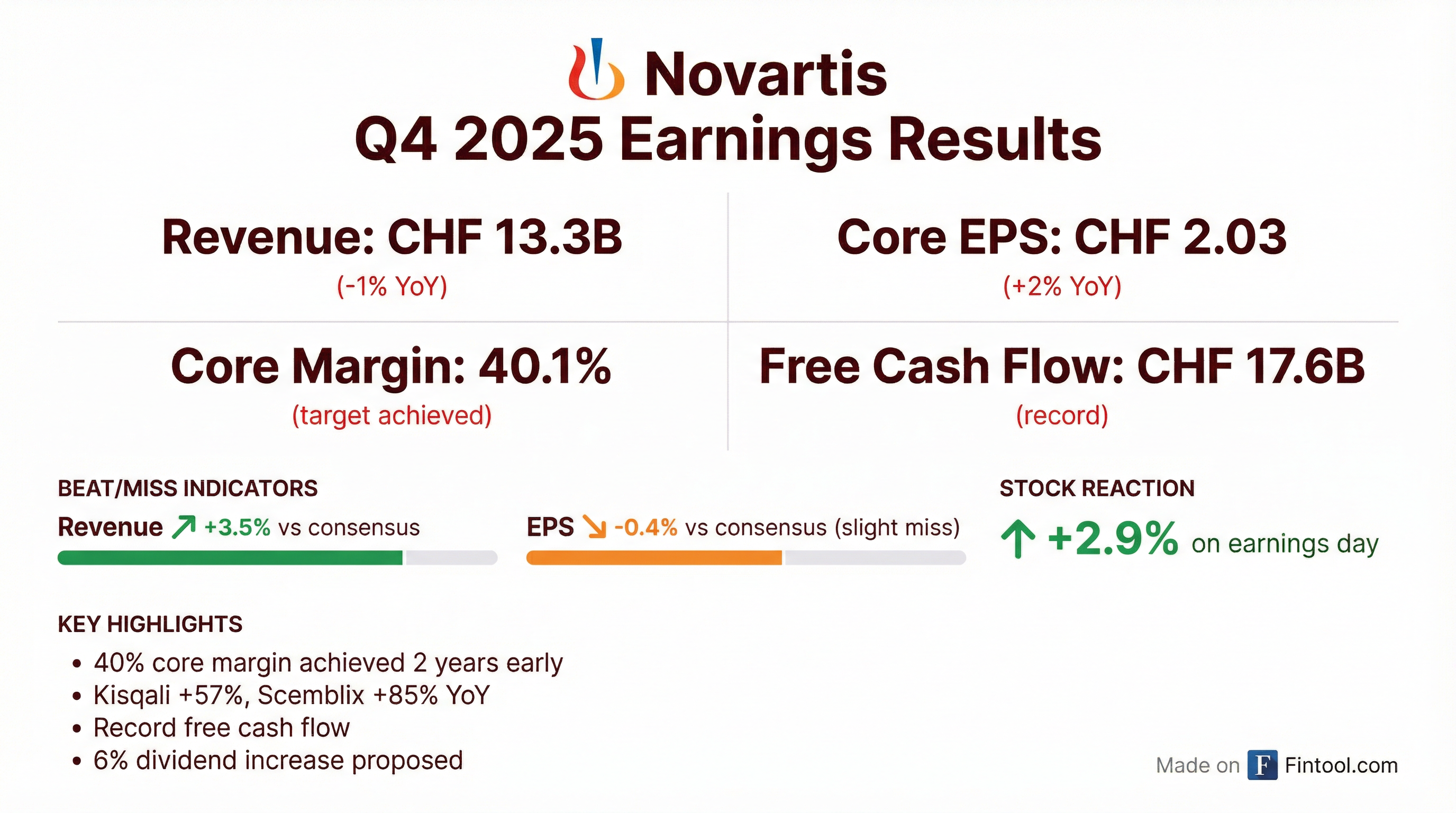
- Novartis delivered strong full-year 2025 results, with sales up 8% and core operating income up 14%, achieving its 40.1% core margin goal two years ahead of schedule.
- Key growth brands significantly contributed to performance, including Kisqali (up 57% to CHF 4.8 billion), Kesimpta (up 36% to $4.4 billion), and Scemblix (up 85%).
- For 2026, the company expects low single-digit sales growth and a low single-digit decline in core operating income, anticipating a stronger second half after initial impacts from generic entries.
- Novartis maintains confidence in a 5%-6% sales CAGR for the 2025-2030 period and aims to return to 40%+ core margin by 2029.
- The company also announced a CFO transition, with Harry Kirsch stepping down and Mukul Mehta taking over in mid-March.
- Novartis delivered high single-digit growth in 2025, with full-year sales up 8% and core operating income up 14%, achieving a 40.1% core margin two years ahead of plan.
- For Q4 2025, sales declined 1% and core operating income increased 1%, impacted by gross-to-net adjustments and Entresto LOE, though underlying Q4 sales growth would have been positive 3% excluding these adjustments.
- The company achieved its upgraded full-year 2025 guidance and expects a 5%-6% sales CAGR from 2025-2030, driven by strong performance from key growth brands such as Kisqali (up 57% full year), Kesimpta (up 36% full year), Scemblix (up 85% full year), and Pluvicto (up 42% constant currency full year).
- Novartis is proposing a dividend of CHF 3.70 per share, representing a 6% increase, marking its 29th consecutive dividend increase.
- Harry Kirsch will transition from his role as Chief Financial Officer, with Mukul Mehta taking over in mid-March 2026.
- Novartis delivered strong full-year 2025 financial results, with 8% sales growth, 14% core operating income growth, $8.98 core EPS (up 17%), and CHF 17.6 billion in free cash flow (up 8%), achieving a 40.1% core margin.
- For 2026, the company expects low single-digit sales growth and a low single-digit decline in core operating income, primarily due to the impact of US generic entries and a 1-2 percentage point core margin dilution from the Avidity deal. The year is anticipated to have a weaker first half followed by a stronger second half.
- The company continues its commitment to shareholder returns, proposing a CHF 3.70 per share dividend (a 6% increase and its 29th consecutive increase) and having CHF 7.7 billion remaining in its CHF 10 billion share buyback program.
- Key product updates include the US approval of Itvisma, which has a total sales potential of $3 billion+ and is expected to ramp up quickly over the next 2-3 years. Additionally, Pelabresib showed strong Phase 3 data, leading to an agreement with the EU to file in 2026.
- Harry Kirsch will be stepping down as CFO, with Mukul Mehta taking over the role.
- Novartis reported full year 2025 net sales of USD 54.5 billion, an 8% increase (8% cc), with core operating income up 14% (cc) to USD 21.9 billion and core EPS of USD 8.98, up 17% (cc).
- Free cash flow for full year 2025 was USD 17.6 billion, an 8% increase, while Q4 2025 free cash flow decreased by 54% to USD 1.7 billion.
- The company provided 2026 guidance expecting net sales to grow low single-digit and core operating income to decline low single-digit, incorporating the impact of an agreement with the US government on drug prices.
- In 2025, Novartis repurchased 77.6 million shares for USD 8.9 billion and proposed a dividend of CHF 3.70 per share for 2025, an increase of 5.7%. Net debt increased to USD 21.9 billion at December 31, 2025.
- Novartis reported strong financial performance, with 7% sales growth and 15% core operating income growth, achieving a 38.7% core operating margin by 2024 and generating $16 billion in free cash flow through Q3 2025.
- The company provided long-term guidance, targeting high single-digit growth for 2024-2025, 5%-6% growth to 2030, and aiming for a 40% core operating margin by 2029.
- Novartis is pursuing a pure-play medicines strategy, underpinned by a deep pipeline featuring 14 in-market blockbusters and 15 submission-enabling readouts in the next two years. Notable assets like Kisqali are now projected for $10 billion+ peak sales, and Scemblix for $4 billion+.
- Capital allocation priorities include consistently growing dividends, completing a $10 billion share buyback this year, and strategic bolt-on acquisitions such as the proposed acquisition of Avidity.
Fintool News
In-depth analysis and coverage of NOVARTIS.
Quarterly earnings call transcripts for NOVARTIS.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more