TAKEDA PHARMACEUTICAL CO (TAK)·Q3 2026 Earnings Summary
Takeda Cuts Revenue Guidance on Vyvanse Erosion; CEO Transition Announced
January 29, 2026 · by Fintool AI Agent
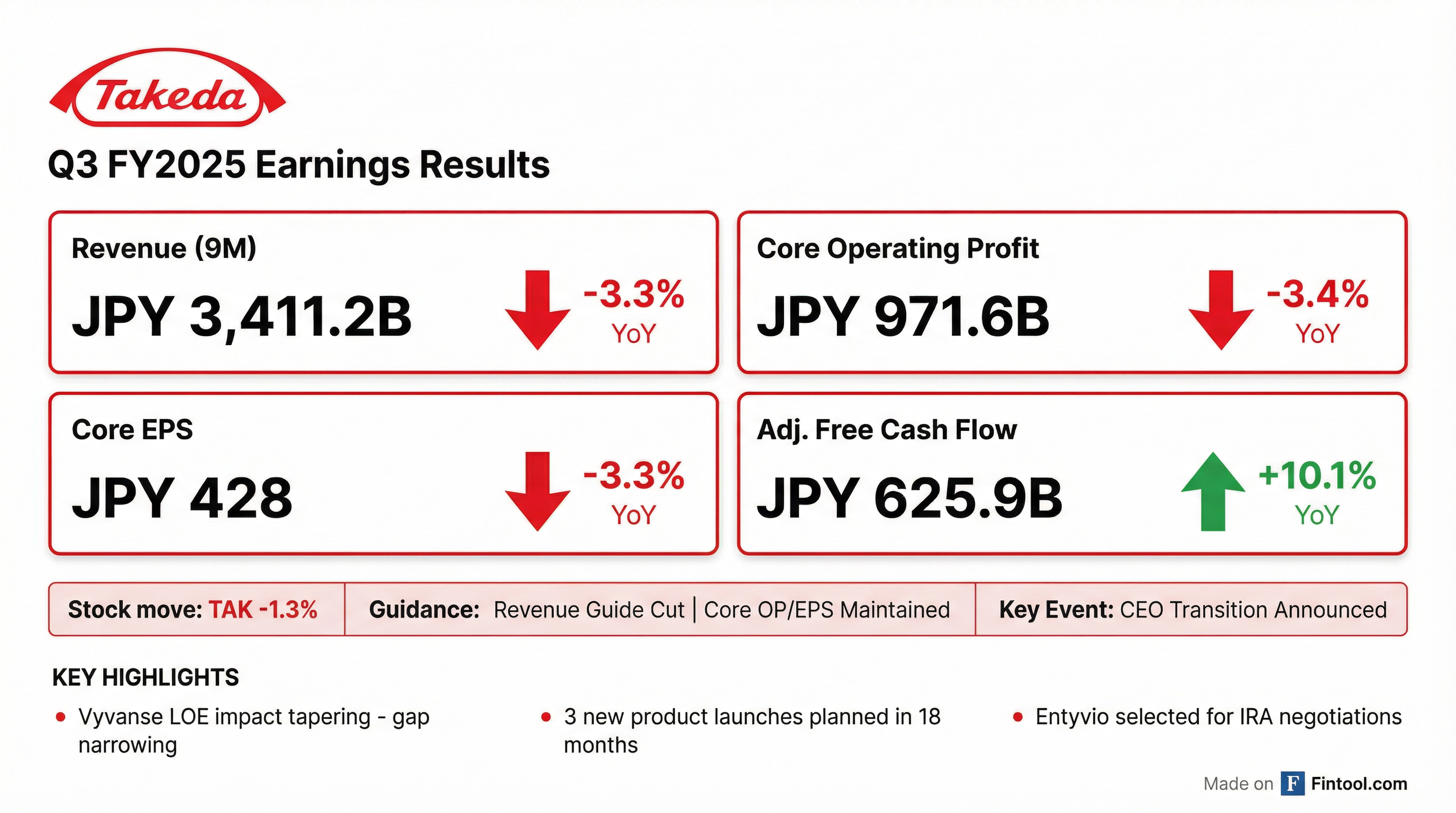
Takeda reported Q3 FY2025 results (nine months ended December 31, 2025) with revenue declining 3.3% YoY to JPY 3,411.2 billion, driven by Vyvanse generic erosion. The company cut its full-year revenue guidance but maintained profit outlook through cost discipline. Shares fell 1.3% to $16.69 on the news.
The quarter was defined by three key developments: (1) the narrowing gap between Vyvanse LOE impact and growth product momentum, (2) the announcement of CEO Christophe Weber's transition to Julie Kim effective June 2026, and (3) positive pipeline readouts setting up three major launches over the next 18 months.
Did Takeda Beat Earnings?
Takeda's results reflected the ongoing Vyvanse headwind, though the impact is moderating:
The standout metric was adjusted free cash flow, which grew 10.1% YoY to JPY 625.9 billion despite the revenue decline. This includes the $1.2 billion upfront payment to Innovent Biologics in December for the licensing deal.
Growth and launch products now represent over 50% of total revenue and grew 6.7% at CER, an improvement from 5% growth in Q1 and Q2.
What Did Management Guide?
Takeda revised its full-year management guidance, cutting revenue outlook while maintaining profit guidance:
The company also raised its full-year forecasts in yen terms due to FX tailwinds:
- Revenue: JPY 4.53 trillion (up JPY 30B from prior forecast)
- Core OP: JPY 1.15 trillion (up JPY 20B)
- Core EPS: JPY 486
CFO Milano Furuta emphasized cost discipline: "We have been able to limit the Vyvanse impact through operational efficiencies with R&D and SG&A expenses, both lower than the prior year."
How Did the Stock React?
Takeda shares fell 1.3% to $16.69 on the earnings release, pulling back from a 52-week high of $16.93 reached earlier in the week. The stock has rallied ~28% from its 52-week low of $12.99, driven by positive pipeline readouts and the pending CEO transition.
The muted reaction suggests the revenue guidance cut was largely expected given ongoing Vyvanse dynamics, while investors focus on the upcoming product launches.
What Changed From Last Quarter?
Several key developments differentiated Q3 from prior quarters:
1. Vyvanse Impact Tapering
The headwind from Vyvanse generics is steadily diminishing quarter-by-quarter. Management noted the gap between growth products and LOE impact is "becoming smaller every quarter."
2. Entyvio Coverage Milestones
Entyvio Pen achieved a critical milestone: as of January 2026, it's now on formulary with all three large PBMs with commercial coverage exceeding 80%, matching competitors.
3. CEO Transition Announced
Christophe Weber announced this is his last earnings call as main presenter. Julie Kim will lead the FY26 guidance call and formally take over as CEO in June 2026.
4. Organizational Restructuring
Effective April 1, Takeda is implementing organizational changes including:
- Maintaining a separate oncology business unit
- Creating two geographic commercial structures (U.S. and International)
- Enhanced marketing and commercial operations capabilities
Three Product Launches in 18 Months
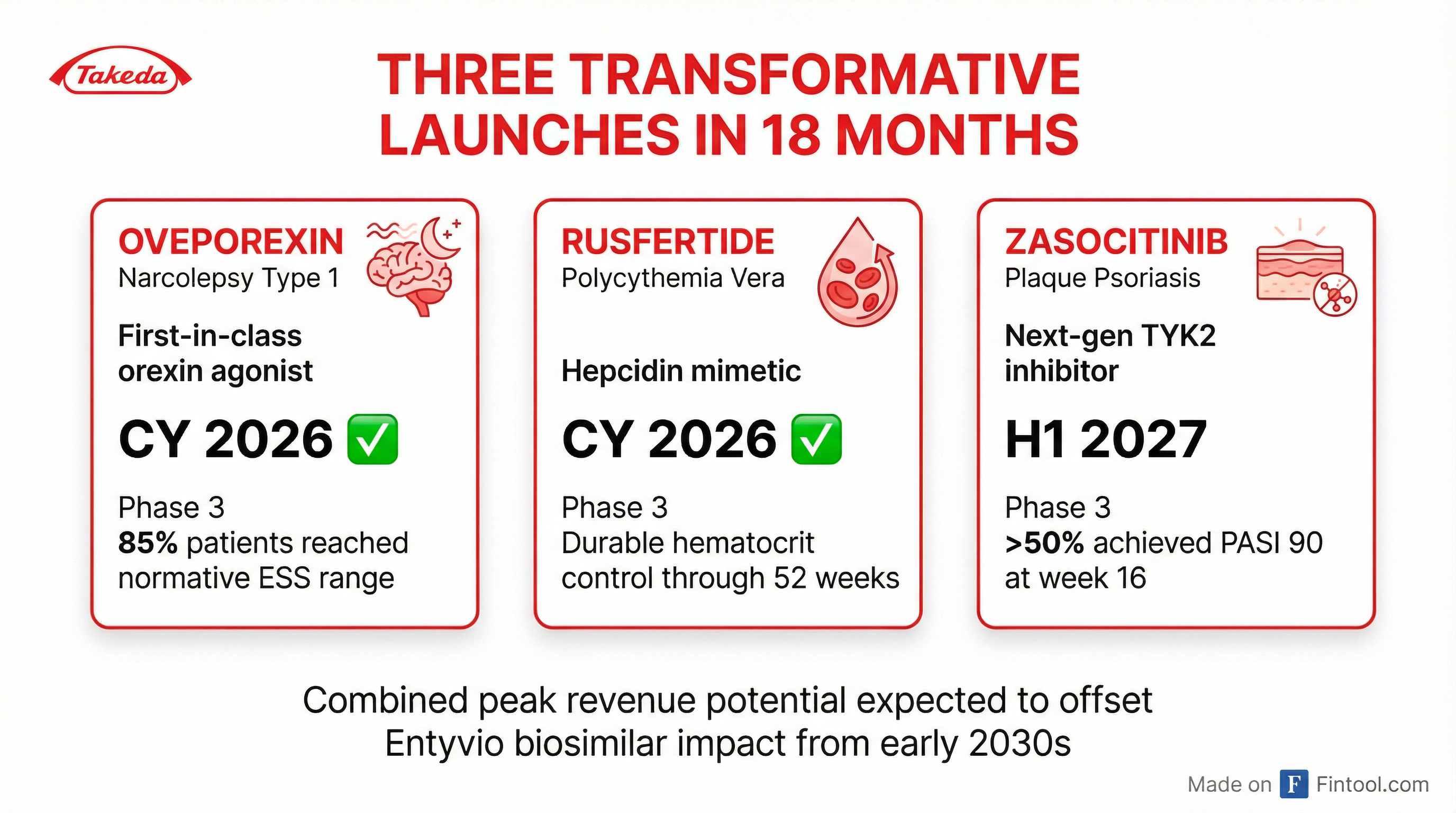
Takeda delivered positive Phase 3 results for all three key pipeline assets in 2025, setting up a transformative launch period:
Oveporexin (Narcolepsy Type 1) — Launch CY 2026
- First-in-class orexin 2 receptor agonist
- Addresses underlying orexin deficiency rather than just managing symptoms
- ~85% of patients achieved normative range on Epworth Sleepiness Scale
- Hit all 14 primary and secondary endpoints in both Phase 3 studies
- NDA submitted to FDA
Rusfertide (Polycythemia Vera) — Launch CY 2026
- Hepcidin mimetic providing durable hematocrit control
- Nearly half of PV patients remain untreated; treated patients still struggle with disease management
- Maintained hematocrit control below 45% through 52 weeks
- Statistically significant quality of life improvements
- NDA submitted to FDA
Zasocitinib (Psoriasis) — Launch H1 2027
- Next-generation TYK2 inhibitor
- >50% achieved PASI 90 (almost clear skin) at week 16
- ~30% achieved PASI 100 (completely clear skin)
- Once-daily oral, well-tolerated, no food interactions
- Filing expected summer 2026
Management emphasized these three products "could more than offset the anticipated impact of Entyvio biosimilar entry from the early 2030s onwards."
Key Q&A Highlights
On FY2026 Outlook (Analyst: Morgan Stanley)
CFO Furuta declined to give specific FY2026 guidance but noted: "Launch costs for three products to be launched within one year means there will be some burden... but this uptick is very important for future growth." He added R&D expenses are "likely to go up" due to Innovent partnership assets entering full-scale development.
On Entyvio IRA Selection (Analyst: Jefferies)
Entyvio was selected for the third cycle of IRA price negotiations, with pricing effective from 2028. CEO-elect Julie Kim noted: "It is too early to say whether [the price cut] trend will continue into the third cohort or whether it will be similar to the second cohort." Peak revenue guidance of $7.5-9 billion may be impacted depending on final pricing.
On Takhzyro Competition (Analyst: Nomura)
Takhzyro growth slowed to 2.4% at CER due to competitive entrants affecting new patient starts. However, Julie Kim emphasized: "From an efficacy standpoint, our real world data for Takhzyro can't be beat. We have patients that are attack-free for over a year."
On Zasocitinib Data Presentation (Analyst: JP Morgan)
R&D President Andy Plump confirmed data will likely be presented at AAD (American Academy of Dermatology) in March, noting: "Watch out for the abstract when they're released in mid-February." He highlighted 44 total endpoints hit across both Phase 3 studies.
Segment Performance
Growth and launch products collectively grew 6.7% at CER, improving from 5% growth in H1.
Forward Catalysts
Risks and Concerns
-
Vyvanse erosion exceeded expectations — Management cut revenue guidance specifically due to stronger-than-anticipated generic impact
-
Entyvio IRA pricing uncertainty — Selection for price negotiations could impact peak revenue potential; second cohort had larger cuts than first
-
China albumin demand softening — Government utilization guidelines impacting demand; full-year forecast at risk
-
Takhzyro competitive pressure — New entrants affecting new patient starts in U.S.
-
Launch execution risk — Three major launches in 18 months requires significant commercial investment
Bottom Line
Takeda's Q3 results reflect a company in transition: absorbing the final phases of Vyvanse LOE while preparing for a transformative launch cycle. The revenue guidance cut was disappointing but expected, while the maintained profit outlook and strong free cash flow demonstrate cost discipline.
The three positive Phase 3 readouts in 2025 — oveporexin, rusfertide, and zasocitinib — position Takeda for growth beyond the Vyvanse headwind. Management's assertion that these launches can offset Entyvio biosimilar risk in the 2030s will be tested over the coming 18 months.
The CEO transition to Julie Kim appears orderly and well-coordinated, with organizational changes already in motion to support the upcoming launch period. Investors will be watching AAD in March for the full zasocitinib data and FDA decisions on oveporexin and rusfertide later this year.