TEVA PHARMACEUTICAL INDUSTRIES (TEVA)·Q4 2025 Earnings Summary
Teva Crushes Q4 on $500M Duvakitug Milestone and AUSTEDO Surge
January 28, 2026 · by Fintool AI Agent
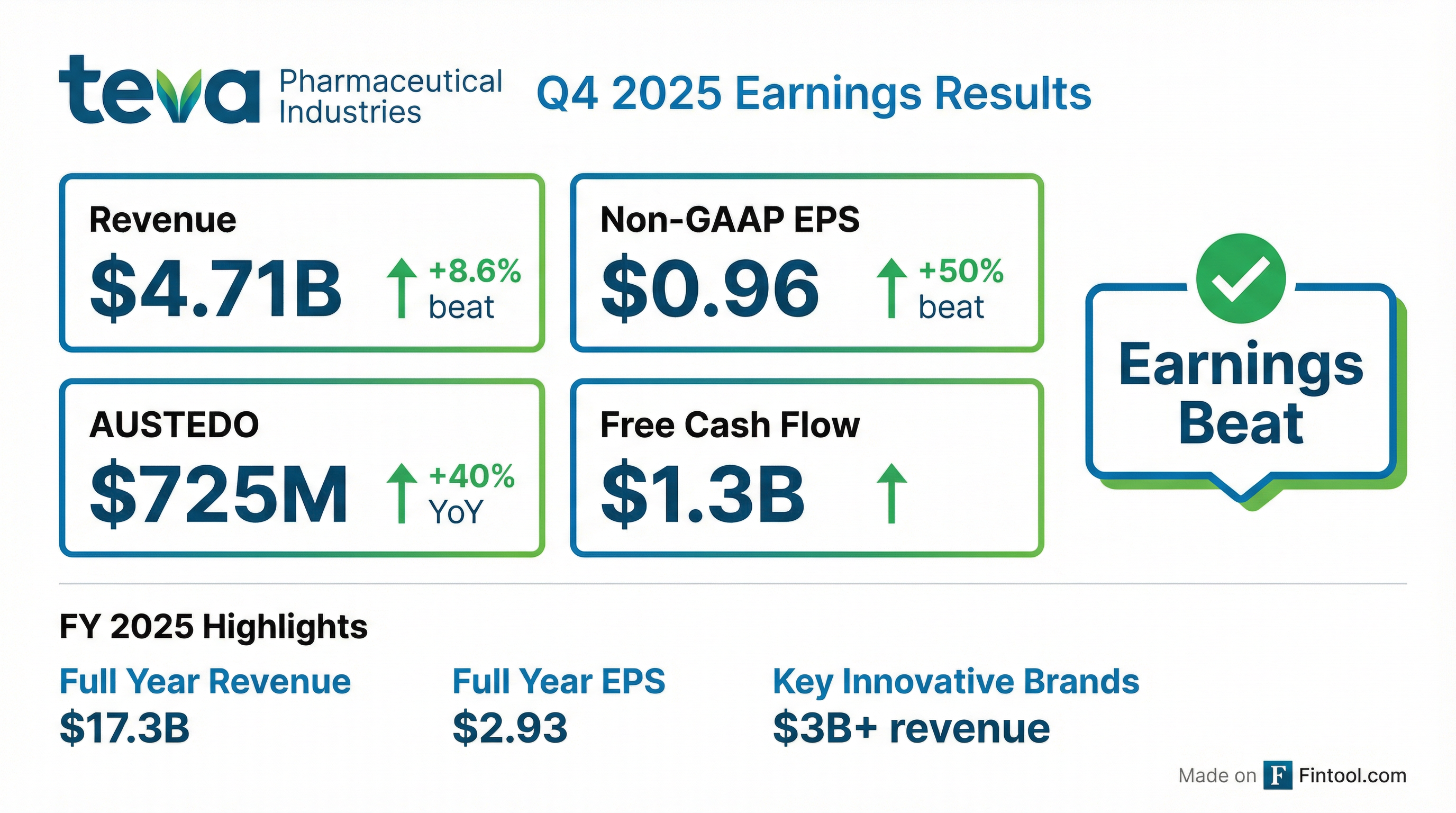
Teva Pharmaceutical delivered a blowout Q4 2025, with Non-GAAP EPS of $0.96 crushing the $0.64 consensus by 50% and revenue of $4.71B beating estimates by 8.6% . The quarter was supercharged by a $500 million milestone payment from Sanofi for initiating Phase 3 studies of duvakitug (anti-TL1A) in ulcerative colitis and Crohn's disease . Key innovative brands collectively surpassed $1 billion in quarterly revenue for the first time, led by AUSTEDO's 40% YoY surge to $725M .
This marks Teva's third consecutive year of growth under the "Pivot to Growth" strategy, cementing its transformation from a generics-dependent company to an innovation-driven biopharma player .
Did Teva Beat Earnings?
Yes — decisively. This was Teva's largest earnings beat in recent memory.
The beat was driven by two factors:
- $500M duvakitug milestone: Recognized upon Phase 3 initiation, this one-time payment added ~$0.30 to EPS
- AUSTEDO momentum: Q4 revenue of $725M (+40% YoY) exceeded expectations, driven by volume growth and reduced sales allowances
Excluding the milestone, revenue was still up ~6% YoY organically — a strong result that validates the innovation pivot.
Teva has now beaten EPS estimates in 8 of the last 9 quarters, with the only miss being Q1 2024's lighter seasonal performance.
What Did Management Guide?
2026 guidance came in below revenue consensus but in-line on EPS.
Key guidance drivers:
- AUSTEDO: $2.4-$2.55B (+6-13% YoY)
- AJOVY: $750-$790M (+11-17% YoY)
- UZEDY: $250-$280M (+31-47% YoY)
The revenue guidance reflects:
- Loss of Revlimid generic sales expected in 2026
- Japan divestiture completed in March 2025 with no contribution going forward
- Medicare price negotiation for AUSTEDO effective January 2027
Management targets 30% Non-GAAP operating margin by 2027, driven by mix shift to higher-margin innovative products and ~$700M in transformation cost savings .
What Changed From Last Quarter?
Key deltas vs Q3 2025:
CEO Richard Francis emphasized the strategic transformation:
"In 2025, our Pivot to Growth strategy drove Teva's third year of consecutive growth, solidifying our transformation into a leading biopharmaceutical company. Our key innovative brands led our growth, reaching $1 billion in revenues in the fourth quarter of 2025 for the first time, and becoming a true engine of sustainable growth."
How Did Key Products Perform?
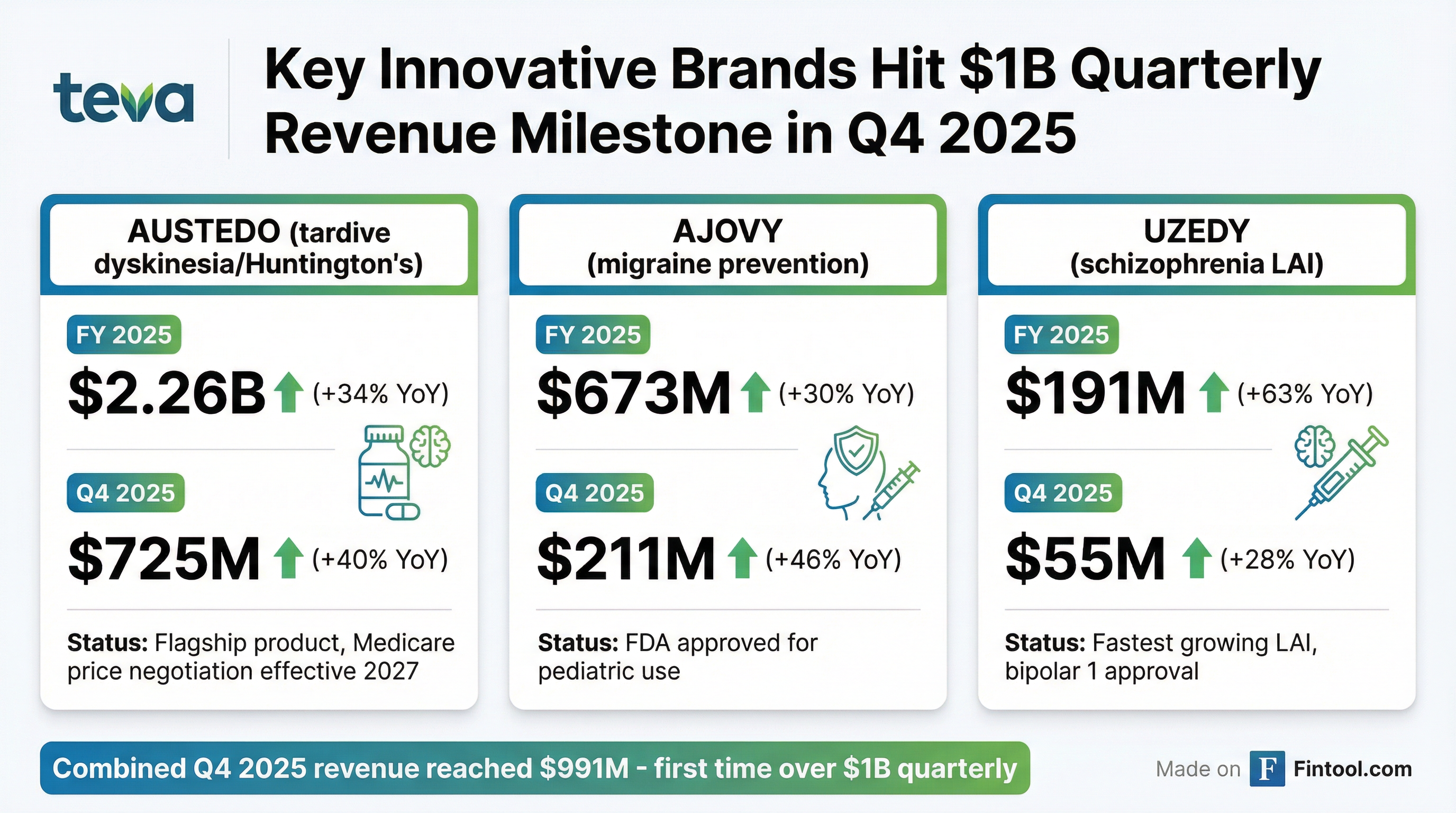
AUSTEDO (Tardive Dyskinesia / Huntington's)
AUSTEDO continues to be the flagship growth driver. The once-daily AUSTEDO XR formulation now accounts for 60% of new patients and is protected by 11 Orange Book patents expiring between 2031 and 2041 . Management expects peak sales above $3B.
CEO Francis emphasized the growth runway: "The most important thing for AUSTEDO is to keep reminding everybody that 85% of people who suffer from Tardive Dyskinesia are still not treated."
Medicare price negotiation: CMS announced a maximum fair price for AUSTEDO effective January 1, 2027 . Impact remains to be quantified but is reflected in 2027+ planning.
AJOVY (Migraine Prevention)
AJOVY gained share in the competitive anti-CGRP migraine market. FDA approved a pediatric indication expansion in 2025 . The team also earned a New England Journal of Medicine publication for their pediatric migraine data .
UZEDY (Schizophrenia LAI)
UZEDY is the fastest-growing long-acting injectable in its category . Notably, more than 83% of new prescriptions came from patients transitioning from other therapies or treatment-naïve patients — confirming UZEDY is expanding the LAI market, not just taking share . FDA approved the Bipolar 1 Disorder indication in 2025 .
What's in the Pipeline?
Teva's late-stage pipeline represents >$10B in potential value according to management .
Duvakitug highlight: Teva secured up to $500M in funding from Royalty Pharma in January 2026 to accelerate the anti-IL-15 vitiligo program .
Biosimilars: Teva has the second-largest biosimilar portfolio in the industry with 18 assets. Recent launches include SELARSDI (Stelara biosimilar) and EPYSQLI (Soliris biosimilar) .
How Is the Balance Sheet?
Deleveraging remains on track. Credit ratings were upgraded one level at three agencies in 2025 . Average debt maturity is 5.6 years .
How Did the Stock React?
Teva reported before market open on January 28, 2026. The stock closed at $32.53 on January 27, up 161% from its 52-week low of $12.47 .
Pre-earnings setup: The stock traded near 52-week highs heading into the report, reflecting optimism around the innovation pivot. Analyst upgrades in 2025 included Goldman Sachs (Buy initiation), Truist Securities (Buy initiation), and JP Morgan (upgrade to Overweight) .
Post-earnings reaction: [Stock reaction pending market open]
What Are the Key Risks?
- Medicare price negotiation for AUSTEDO effective 2027 — revenue impact unknown
- Revlimid generic headwinds in 2026 as volume declines
- Opioid litigation payments ongoing (~$220M provision update in 2025)
- Tariff uncertainty — guidance assumes current trade environment
- Pipeline execution — duvakitug Phase 3 reads critical for long-term value
What Did Management Say in Q&A?
On AUSTEDO Q4 Dynamics — CEO Richard Francis clarified the one-time benefits:
"The vast majority, pretty much, was the inventory. So that's why obviously we have a lot of confidence about 2026 on our numbers."
The $100M benefit was primarily inventory stocking, with minimal gross-to-net benefit. Backing out these one-time items, AUSTEDO still grew ~20% in Q4 and the 2026 guidance range implies 11%-18% underlying growth .
On Pipeline External Validation — CMO Eric Hughes highlighted third-party recognition:
"We've had external validation on four of these five programs. Olanzapine LAI got Royalty Pharma funding. Duvakitug was partnered with Sanofi. The DARI program was acknowledged by Abingworth. The Anti-IL-15 program was recently acknowledged again by Royalty Pharma."
On BD Strategy — Management signaled increased inbound interest:
"What is interesting, I think within the last year to 18 months, the amount of approaches we've had has significantly increased. And I think that's because they see Teva as a partner, both from an R&D perspective, the speed which we move things through the clinic is exciting, but also primarily because of the commercial capability and muscle we have."
On Duvakitug Differentiation — The company believes it has best-in-class TL1A:
"We stand by the fact that we have, and we believe we have the best TL1A."
How Should We Think About Quarterly Cadence?
Q1 2026 will be light. Management flagged two headwinds :
- Revlimid loss: ~$300M in generic Revlimid revenue from Q1 2025 that won't repeat
- AUSTEDO sequential decline: Q4 benefited from ~$100M in inventory build and gross-to-net timing
Q4 2026 AUSTEDO risk: Management warned that Q4 2026 revenue could be "potentially down year-over-year due to different purchasing patterns and pricing environment ahead of the IRA implementation in January 2027" .
Margin trajectory: Non-GAAP margins expected to "gradually ramp up over the course of the year" as transformation savings accelerate in H2 .
Biosimilar Launch Details
Management provided specifics on upcoming launches :
The company has 10 more biosimilar assets in the pipeline launching from 2028 onwards, reinforcing its position as the second-largest biosimilar portfolio in the industry .
What Should Investors Watch Next?
2026 R&D Catalysts — Seven milestones expected :
Additional Duvakitug Indications: Management announced plans to disclose two new indications for duvakitug later in 2026, highlighting it as a "pipeline in a product" .
Related: Teva Company Profile | Q3 2025 Earnings | All Teva Transcripts