Axsome Surges 20% to All-Time High on FDA Priority Review for Alzheimer's Drug
December 31, 2025 · by Fintool Agent
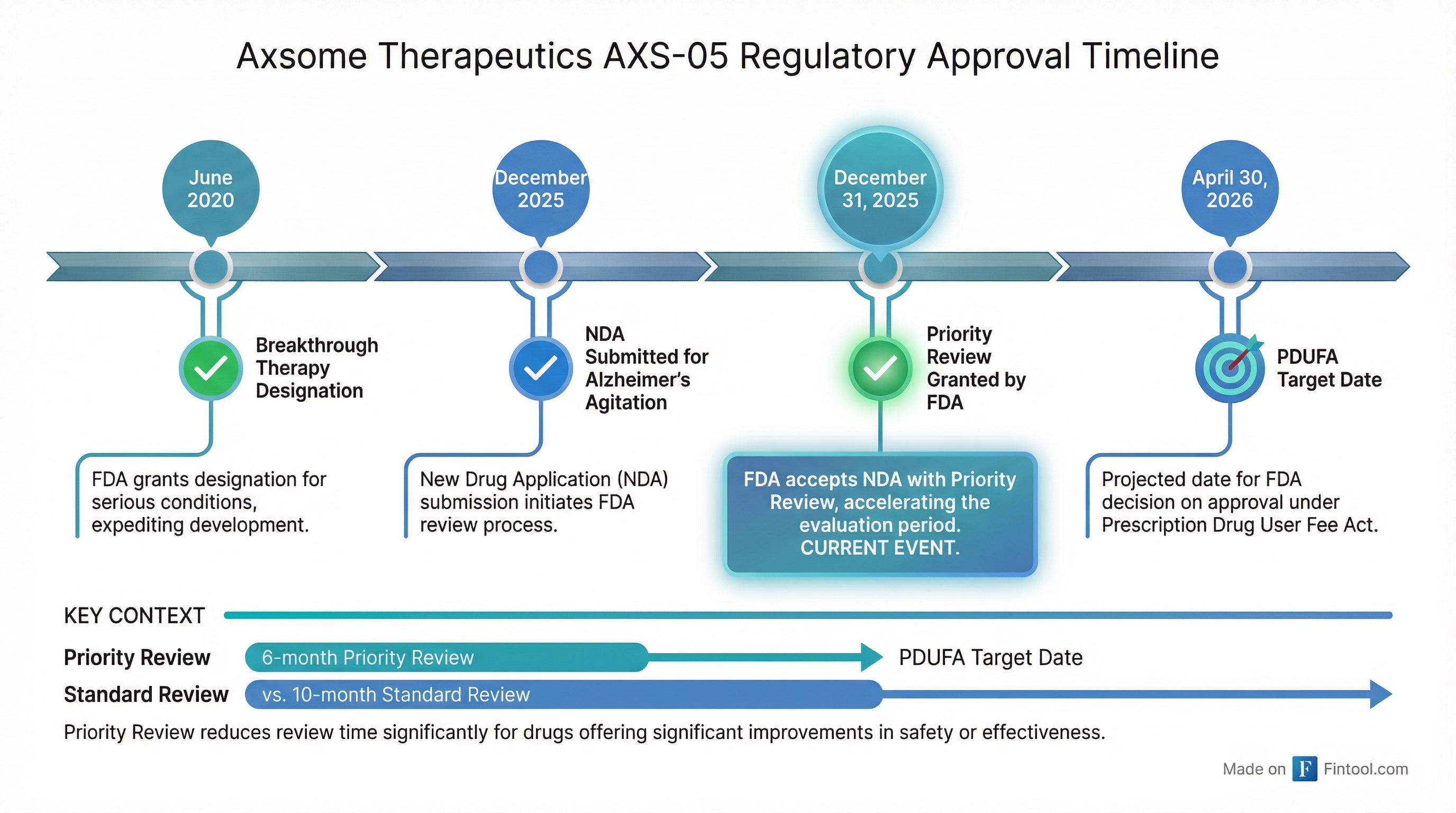
Axsome Therapeutics is closing out 2025 with a bang. Shares surged more than 20% to an all-time high of $179.98 on the final trading day of the year after the FDA granted Priority Review to the company's supplemental New Drug Application for AXS-05 (Auvelity) for the treatment of Alzheimer's disease agitation.
The FDA has set a PDUFA target action date of April 30, 2026—giving investors a clear catalyst on the calendar for the first half of next year.
The Double Catalyst
Today's rally wasn't driven by a single announcement. Axsome delivered a one-two punch of regulatory news that sent the stock rocketing:
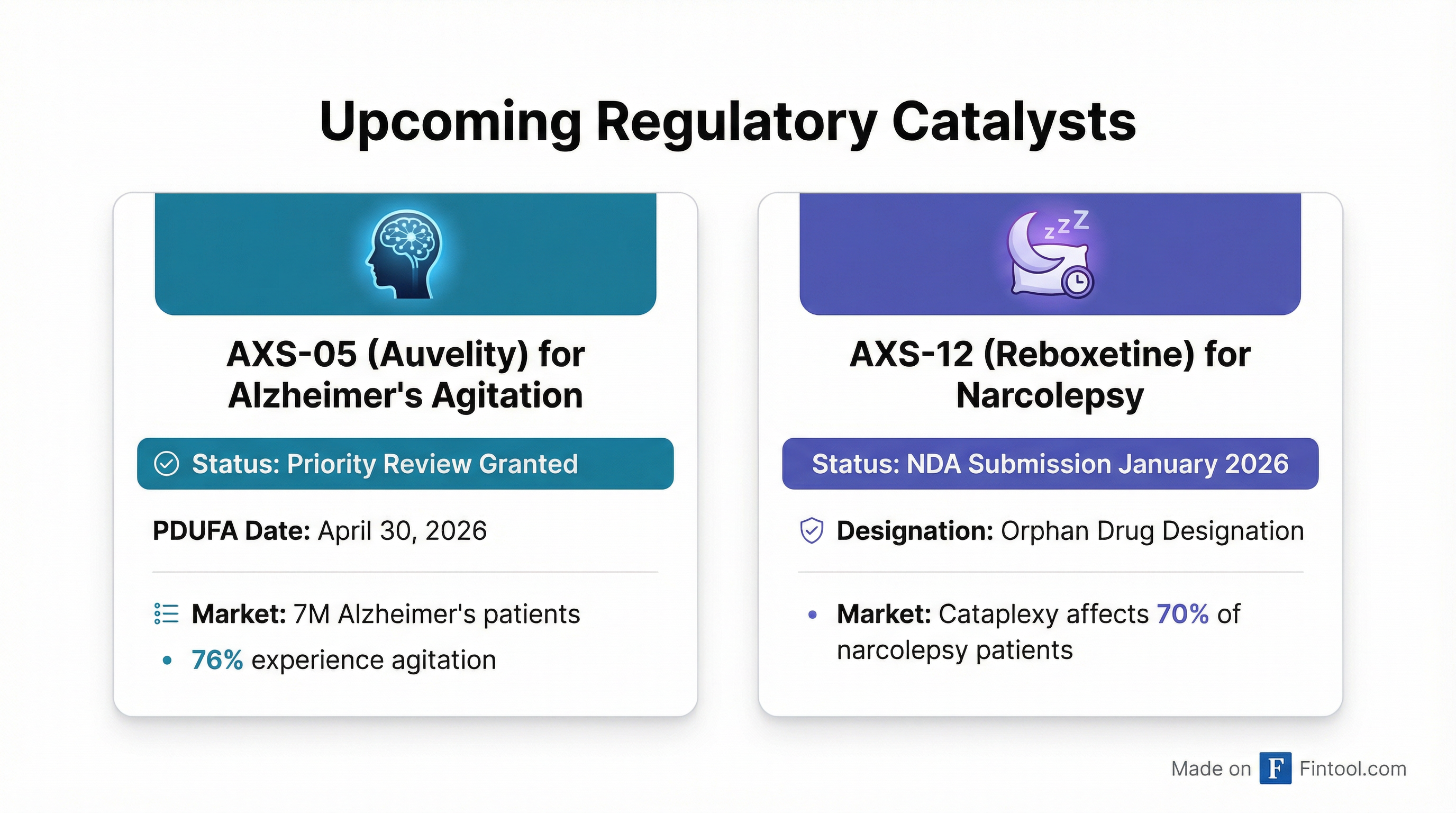
1. Priority Review for Alzheimer's Agitation — The FDA accepted Axsome's supplemental NDA for AXS-05 and granted Priority Review designation, which means the agency's goal is to take action within 6 months rather than the standard 10 months. Priority Review is reserved for drugs that, if approved, would provide significant improvements in treatment, diagnosis, or prevention of serious conditions.
2. Green Light for Narcolepsy NDA — In a separate announcement, Axsome disclosed that it received positive FDA pre-NDA meeting minutes supporting submission of its AXS-12 (reboxetine) application for the treatment of cataplexy in narcolepsy. The company now expects to complete the NDA submission in January 2026.
The Alzheimer's Agitation Opportunity
The market for Alzheimer's disease agitation is substantial and severely underserved. Approximately 7 million people in the United States have Alzheimer's disease, and up to 76% of them experience agitation—characterized by emotional distress, verbal and physical aggressiveness, disruptive irritability, and disinhibition.
"Currently there is a dearth of approved treatments," CEO Herriot Tabuteau noted in the press release.
Alzheimer's agitation isn't just uncomfortable—it's dangerous. The condition has been associated with accelerated cognitive decline, increased caregiver burden, earlier nursing home placement, and increased mortality.
The FDA previously granted Breakthrough Therapy designation for AXS-05 for Alzheimer's agitation in June 2020, signaling early confidence in the drug's potential.
What Is AXS-05?
AXS-05 (dextromethorphan-bupropion), sold commercially as Auvelity for major depressive disorder, is a novel oral NMDA receptor antagonist and sigma-1 agonist. The dextromethorphan component modulates glutamate receptors, while bupropion increases bioavailability and provides norepinephrine and dopamine reuptake inhibition.
The supplemental NDA is backed by a comprehensive clinical development program including four randomized, double-blind, controlled Phase 3 clinical trials and a long-term safety trial.
Narcolepsy: The Second Pillar
AXS-12 (reboxetine) represents another significant opportunity. The drug is a highly selective norepinephrine reuptake inhibitor under development for narcolepsy, and it has already been granted Orphan Drug Designation—which could provide seven years of marketing exclusivity upon approval and a waiver of FDA application user fees.
Cataplexy—sudden muscle weakness triggered by strong emotions—affects approximately 70% of narcolepsy patients and represents a life-altering symptom with limited treatment options.
Financial Trajectory
Axsome's commercial execution has been impressive. The company's revenue has grown consistently as Auvelity gains traction in the depression market:
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenue (USD Millions) | $118.8 | $121.5 | $150.0 | $171.0 |
| Gross Margin | 91.1% | 91.9% | 91.0% | 93.0% |
| Net Income (USD Millions) | ($74.9) | ($59.4) | ($48.0) | ($47.2) |
Revenue grew 44% from Q4 2024 to Q3 2025, and losses have been narrowing consistently as the commercial business scales. The company reported 93% gross margins in Q3 2025.
Analyst Reaction
Wall Street moved quickly to update price targets following the news. Needham reiterated its buy rating and raised its price target to $169 from $154. TD Cowen also maintained its buy rating on the stock.
What to Watch
The April 30, 2026, PDUFA date now becomes the primary catalyst for Axsome investors. Approval would unlock a significant new indication for Auvelity in a market with substantial unmet need.
In the near term, watch for:
- January 2026 — AXS-12 NDA submission for narcolepsy
- April 30, 2026 — PDUFA target date for AXS-05 in Alzheimer's agitation
- Auvelity sales momentum — Continued growth in the MDD indication
With a market cap now approaching $9 billion and a clear regulatory path forward, Axsome enters 2026 as one of the most closely watched names in CNS therapeutics.
Related: Axsome Therapeutics Company Profile