Corcept's Ovarian Cancer Drug Extends Lives by 4 Months in Pivotal Trial, Stock Whipsaws
January 22, 2026 · by Fintool Agent

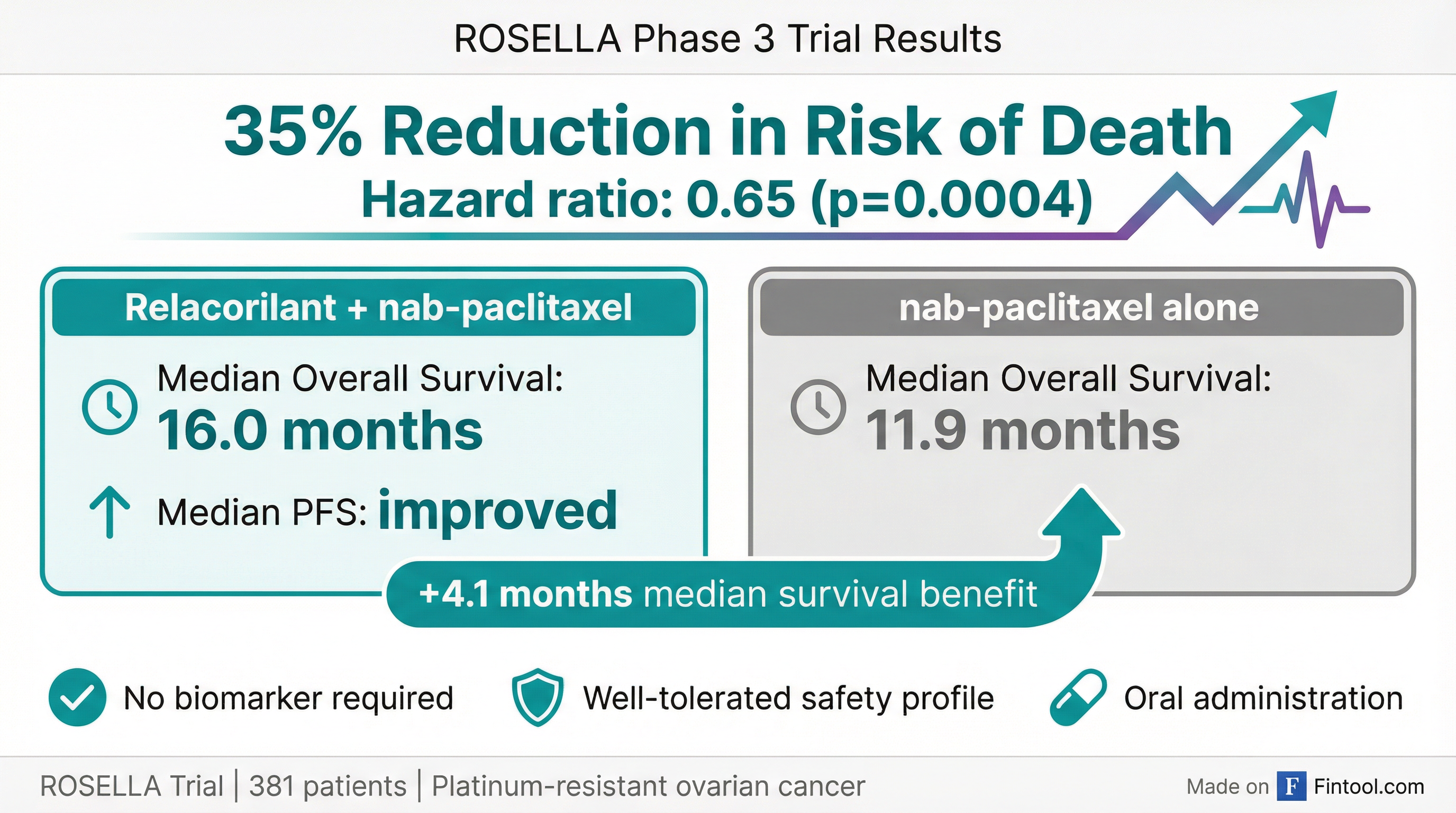
Corcept Therapeutics delivered welcome news to investors still reeling from a surprise FDA rejection three weeks ago: its experimental drug relacorilant significantly extended survival in women with platinum-resistant ovarian cancer, cutting the risk of death by 35%.
The Phase 3 ROSELLA trial showed patients receiving relacorilant plus nab-paclitaxel chemotherapy lived a median of 16.0 months compared to 11.9 months for those on chemotherapy alone—a 4.1-month improvement that investigators say could establish a new standard of care for this notoriously difficult-to-treat cancer.
A Wild Ride for Investors
The positive data sent Corcept shares surging as much as 45% in premarket trading to $52.84, before a dramatic pullback left the stock closing at $41.30—up 14% on the day but still nursing a 41% decline from December levels.
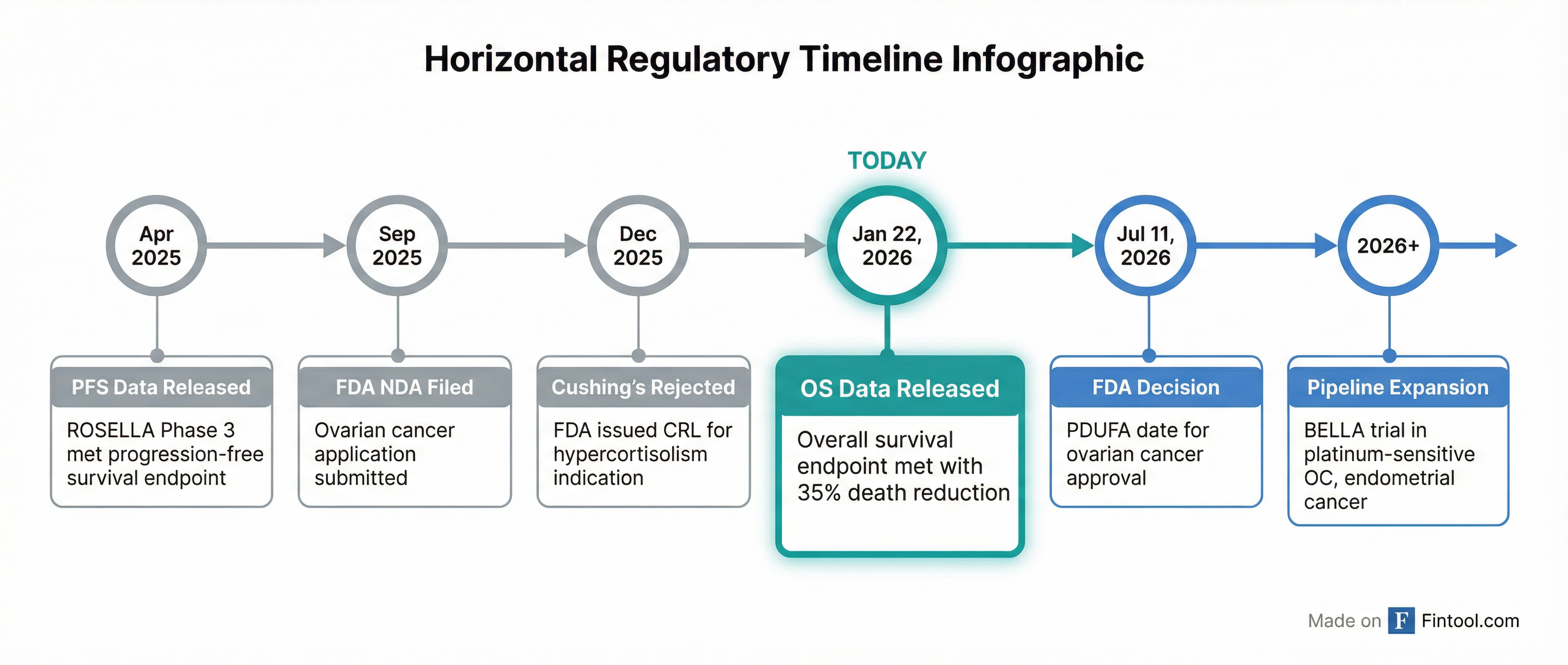
The whipsaw reflects investor uncertainty following the FDA's December 31 Complete Response Letter for the same drug in Cushing's syndrome—a rejection that halved the company's market value overnight.
The Data: Dual Primary Endpoints Met
ROSELLA enrolled 381 patients with platinum-resistant ovarian cancer across sites in the United States, Europe, South Korea, Brazil, Argentina, Canada, and Australia. The trial met both co-primary endpoints:
| Endpoint | Relacorilant + nab-paclitaxel | nab-paclitaxel Alone | Improvement |
|---|---|---|---|
| Median Overall Survival | 16.0 months | 11.9 months | +4.1 months |
| Reduction in Death Risk | — | — | 35% (HR: 0.65, p=0.0004) |
| Reduction in Progression Risk | — | — | 30% (HR: 0.70, p=0.008) |
Crucially, the benefit came without increased toxicity. "The type, frequency and severity of adverse events in the combination arm were comparable to those in the nab-paclitaxel monotherapy arm," Corcept stated.
"The addition of relacorilant to nab-paclitaxel is positioned to become a new standard-of-care treatment for patients with platinum-resistant ovarian cancer, due to its overall survival benefit, well tolerated side effect profile and oral administration," said Dr. Alexander Olawaiye, Director of gynecological cancer research at Magee-Women's Hospital and the trial's principal investigator.
Why This Matters: A Decade of Stagnation
Platinum-resistant ovarian cancer—defined as disease that progresses within six months of platinum-based chemotherapy—has seen almost no treatment advances in over a decade.
Approximately 20,000 women in the U.S. and a comparable number in Europe face this diagnosis annually, with standard chemotherapy producing median survival of roughly one year.
Until last year, the only recent approval in this space was mirvetuximab soravtansine—but that requires folate receptor alpha expression, limiting its applicability. Relacorilant requires no biomarker selection, potentially reaching a broader patient population.
The Mechanism: Cortisol's Role in Cancer
Relacorilant works through an unconventional mechanism—it's a selective glucocorticoid receptor antagonist that blocks cortisol's effects on tumors.
Cortisol plays multiple roles in cancer progression:
- Chemotherapy resistance: Cortisol inhibits cellular apoptosis, reducing chemotherapy's tumor-killing effect
- Tumor growth promotion: In some cancers, cortisol activates oncogenes
- Immune suppression: Elevated cortisol weakens the body's ability to fight disease
By blocking the glucocorticoid receptor, relacorilant essentially re-sensitizes tumors to chemotherapy—a strategy that sets it apart from antibody-drug conjugates and immunotherapies dominating oncology development.
The Bigger Picture: From Cushing's to Cancer
Corcept built its business on Korlym, the only FDA-approved treatment for Cushing's syndrome (hypercortisolism). That franchise generated approximately $675 million in 2024 revenue and is growing rapidly—prescriptions doubled year-over-year in early 2025 as physicians increasingly screen for the condition.
| Metric | Q4 2023 | Q1 2024 | Q2 2024 | Q3 2024 | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|---|---|---|---|
| Revenue ($M) | $135 | $147 | $164 | $183 | $182 | $157 | $194 | $208 |
Relacorilant was expected to supercharge this business, with management projecting $3–5 billion in annual revenue for hypercortisolism alone. The December FDA rejection dealt a significant blow to those aspirations, though Corcept said it will seek a path forward with regulators.
The oncology opportunity may now be even more important. Analysts estimate the platinum-resistant ovarian cancer market alone represents a $1+ billion opportunity, with additional potential in earlier-line ovarian cancer and other solid tumors.
What's Next: The Path to Approval
The FDA has a Prescription Drug User Fee Act (PDUFA) target date of July 11, 2026 for relacorilant in platinum-resistant ovarian cancer. The European Medicines Agency is also reviewing a Marketing Authorization Application.
With both dual primary endpoints met and a clean safety profile, the regulatory path appears straightforward—barring unexpected manufacturing or labeling issues.
Beyond approval, Corcept is expanding its oncology ambitions:
- BELLA trial: Phase 2 study of relacorilant plus nab-paclitaxel and bevacizumab in platinum-resistant and platinum-sensitive ovarian cancer, plus endometrial cancer
- Other solid tumors: Ongoing studies in cervical, pancreatic, and prostate cancers
The Investment Case
Corcept trades at a steep discount to analyst price targets, which average $94 versus the current $41.* The disconnect reflects Cushing's rejection uncertainty, but the oncology data may begin to close that gap.
| Metric | FY 2024 | FY 2025E | FY 2026E |
|---|---|---|---|
| Revenue ($M) | $675* | $805* | $1,056* |
| EPS | $1.23* | $0.80* | $0.61* |
| Analysts Covering | 3* | 5* | 5* |
*Values retrieved from S&P Global
The declining EPS estimates reflect R&D investment as Corcept prepares for potential launches. A successful ovarian cancer approval in July could reset expectations significantly higher.
The Bottom Line
Today's data represent a potential second chance for relacorilant and for Corcept's transformation from a single-product Cushing's company to a diversified oncology player. The 35% reduction in death risk is among the most meaningful survival improvements in platinum-resistant ovarian cancer in years—achieved without the biomarker restrictions or increased toxicity that limit other novel therapies.
The July FDA decision looms as the next major catalyst. With the December Cushing's rejection still fresh, investors appear cautious about getting ahead of regulatory risk. But if relacorilant wins approval for ovarian cancer, the stock's discount to intrinsic value may prove short-lived.