Denali Posts Best-in-Class Sanfilippo Data at WORLD Symposium, Eyes Back-to-Back Rare Disease Approvals
February 5, 2026 · by Fintool Agent

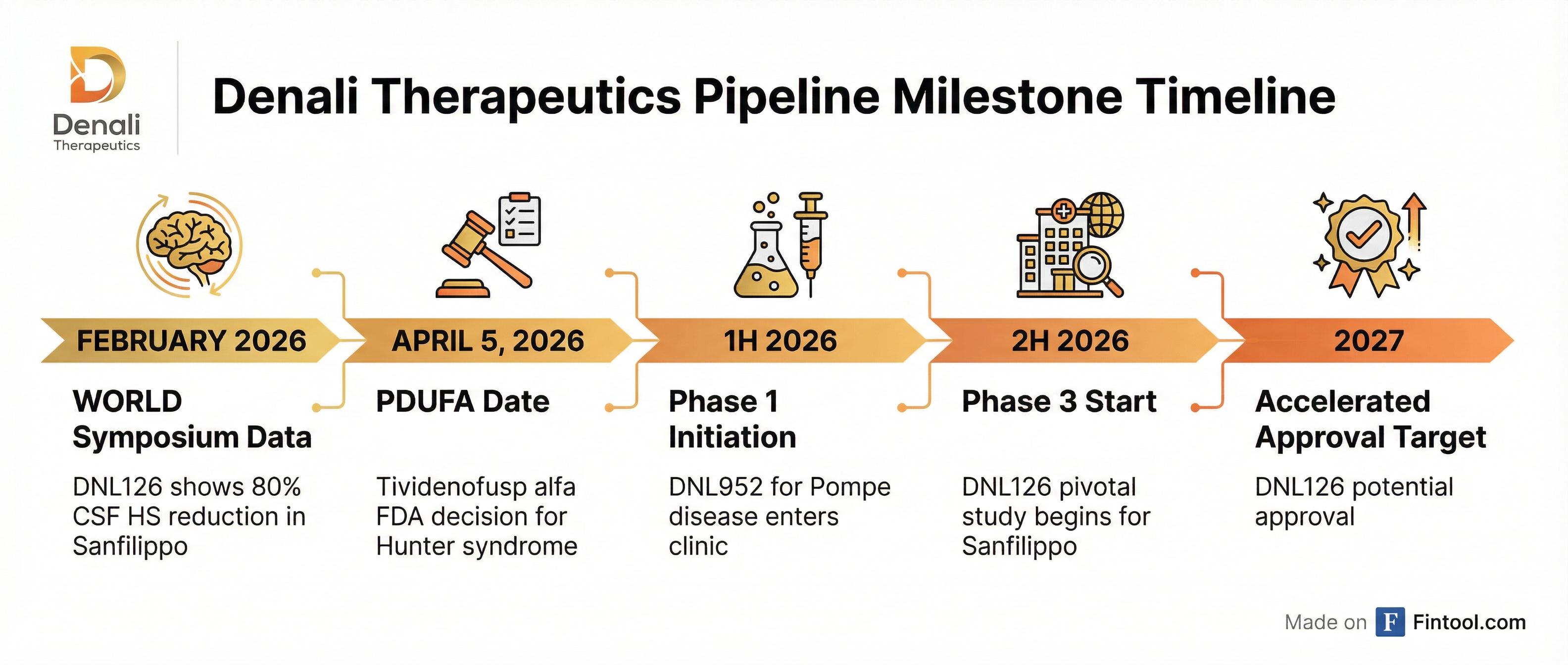
Denali Therapeutics-5.92% unveiled what management called "best-in-class" clinical data for its Sanfilippo syndrome drug at the 2026 WORLDSymposium in San Diego, showing an 80% mean reduction in CSF heparan sulfate—the largest ever reported for this fatal pediatric disease . The biotech is now targeting accelerated FDA approval in 2027 for DNL126 while simultaneously preparing to launch its first commercial product, tividenofusp alfa for Hunter syndrome, pending an April 5 FDA decision .
Shares fell 5.9% to $20.02 on Thursday despite the positive data, extending a three-day pullback from recent highs near $24.
The Sanfilippo Story: 80% Reduction in Key Biomarker
DNL126 delivered robust preliminary results from its phase 1/2 study in 14 children with Sanfilippo syndrome type A (MPS IIIA), a devastating lysosomal storage disorder that causes severe neurodegeneration and death in adolescence .
Key findings at week 49 :
| Biomarker | Reduction | Notes |
|---|---|---|
| CSF Heparan Sulfate | 80% mean reduction | 3 of 7 patients normalized |
| CSF GM3 (lysosomal marker) | 60% reduction | 6 of 7 patients in normal range |
| Urine Heparan Sulfate | 83% reduction | Rapid reduction by week 3 |
| Liver Volume | Normalized | All patients at week 49 |
"This appears to be the largest magnitude of CSF heparan sulfate reduction reported in this very difficult-to-treat disease," CEO Ryan Watts said on the analyst call . The safety profile was consistent with established enzyme replacement therapies, with infusion-related reactions as the most common adverse event—decreasing in frequency and severity over time .
The Competitive Race in Sanfilippo
Denali is not alone in the Sanfilippo space. Ultragenyx-3.65% just resubmitted its BLA for UX111 (rebisufligene etisparvovec), a one-time gene therapy that the company says showed a +23.2 point treatment effect in cognitive scores versus natural history .
When asked about the competitive dynamic, Watts suggested the two approaches may ultimately complement each other: "We imagine that there will be room for both and likely in each patient as gene therapy may wane" .
| Approach | Denali DNL126 | Ultragenyx UX111 |
|---|---|---|
| Mechanism | Weekly IV enzyme replacement | One-time gene therapy |
| CSF HS Reduction | 80% at week 49 | Not directly comparable |
| BLA Status | Targeting 2027 filing | Resubmitted Jan 2026 |
| Key Advantage | Repeat dosing, titration | One-time treatment |
The FDA has selected DNL126 for its START pilot program intended to accelerate rare disease therapy development .
Tividenofusp Alfa: Launch Readiness Ahead of April FDA Decision
While Sanfilippo grabbed headlines, the more immediate catalyst remains tividenofusp alfa (DNL310) for Hunter syndrome. The company presented updated phase 1/2 data showing sustained benefits through 4+ years of follow-up .
Key findings from extended follow-up :
- Biomarkers: CSF and urine heparan sulfate reductions maintained through 201 weeks
- Cognitive function: Younger patients (<4 years at treatment) showed continuous skill gains; older patients maintained stable cognitive scores over nearly 4 years
- Hearing: Improvements in hearing threshold maintained through week 201
- Liver volume: Normal volumes maintained at week 153
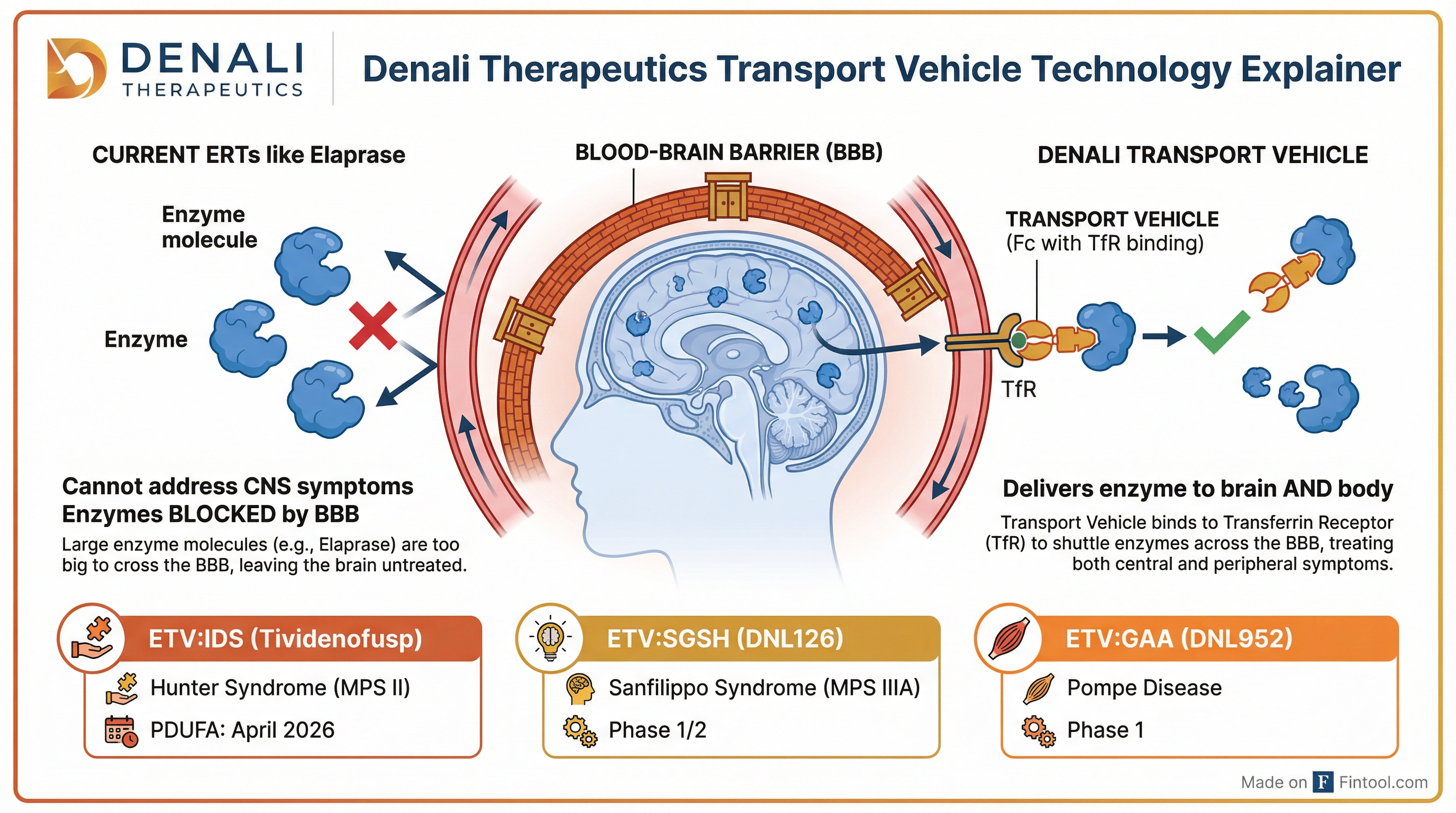
The therapy is designed to address a critical limitation of Takeda's+0.34% Elaprase—the ~$610 million/year Hunter syndrome franchise that cannot cross the blood-brain barrier . Elaprase treats somatic symptoms but leaves the neurological manifestations of the disease—which affect an estimated two-thirds of patients—largely unaddressed .
"The urinary GAG data showing normalization, including in those patients who were already treated with Elaprase with no washout... I think those are highly compelling data," said Acting CMO Peter Chin when asked what would drive switching from Elaprase .
Pompe Disease: Preclinical Data Show Differentiation
Denali also presented Phase 1 study design and preclinical data for DNL952, its Transport Vehicle-enabled therapy for Pompe disease. Chief Scientific Officer Joe Lewcock highlighted that DNL952 showed "substantial reduction of glycogen accumulation" in both muscle and nervous system in mouse models—superior to the same dosing regimen with standard-of-care ERT .
"At all dose levels, [DNL952] is superior to the level of glycogen reduction using the same dosing regimen with 20 mg per kg of the standard of care," Lewcock said .
The Phase 1 study is recruiting adult patients with late-onset Pompe disease, with biomarker proof-of-concept data expected in 2027 .
Financial Position and Investor Considerations
Denali remains pre-revenue but has secured significant financing ahead of its first potential commercial launch. In December 2025, the company announced a $275 million royalty funding agreement with Royalty Pharma tied to tividenofusp alfa's commercial success .
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Net Loss | $115M | $133M | $124M | $127M |
| Cash & Equivalents | $175M | $57M | $141M | $91M |
| Total Assets | $1.37B | $1.27B | $1.17B | $1.06B |
Analysts expect revenue to inflect in 2026 with the potential launch of tividenofusp alfa, projecting $62.7 million in FY 2026 versus essentially no revenue in FY 2025.*
What to Watch
- April 5, 2026: PDUFA decision on tividenofusp alfa for Hunter syndrome—the binary event
- 2H 2026: Phase 3 initiation for DNL126 in Sanfilippo; study design expected to be finalized shortly
- Commercial execution: Insurance coverage and switching dynamics from Elaprase will be critical for tividenofusp alfa's commercial success
- Competitive dynamics: Ultragenyx's UX111 review decision could arrive in mid-2026
*Values retrieved from S&P Global.
Related: