Ironwood Surges 27% on Counter-Intuitive Move: Lower Drug Prices, Higher Net Sales
January 2, 2026 · by Fintool Agent

Ironwood Pharmaceuticals shares exploded 27% on the first trading day of 2026, with volume surging to 40x normal levels, after the company unveiled an unexpectedly strong 2026 outlook powered by a pricing strategy that other drugmakers may soon copy.
The key insight: by cutting LINZESS's list price, Ironwood expects to increase net sales by roughly $265-285 million—a 30%+ jump—because the lower sticker price eliminates mandatory inflationary rebates that have been eroding margins since Medicaid's rebate cap was removed in 2024.
The stock hit an intraday high of $5.78 (up 72% from the prior close) before settling at $4.27—still a 26.7% gain and the biggest single-day move for IRWD in over a year.
The Math Behind the Move
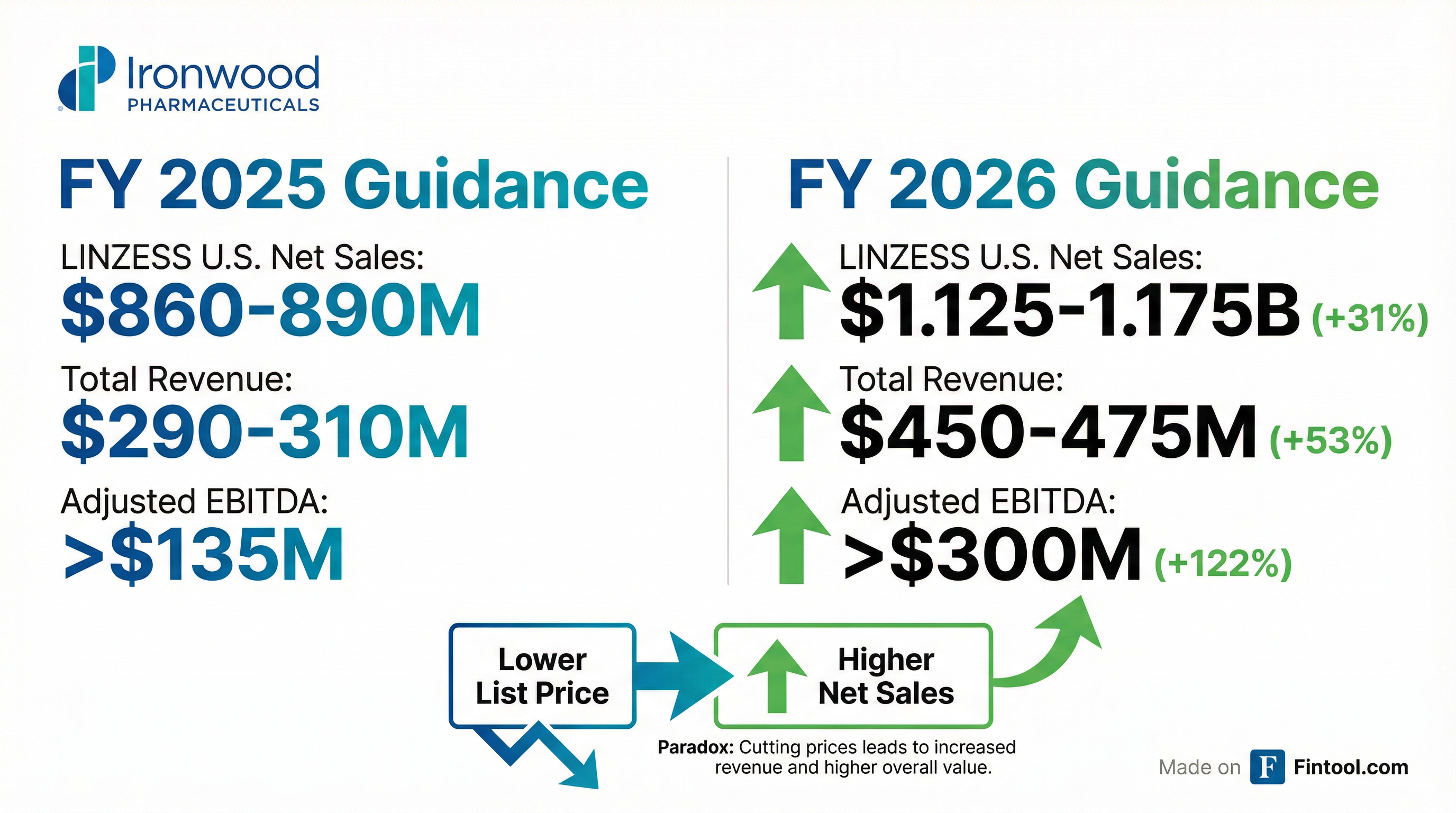
The market wasn't expecting this. Ironwood's 2026 guidance blew past implicit expectations across every metric:
| Metric | FY 2025 Guidance | FY 2026 Guidance | Change |
|---|---|---|---|
| LINZESS U.S. Net Sales | $860-890M | $1.125-1.175B | +31% |
| Total Revenue | $290-310M | $450-475M | +53% |
| Adjusted EBITDA | >$135M | >$300M | +122% |
This isn't growth from new indications or higher volumes—management explicitly stated the gains come from "improved net price and low-single digit percentage demand growth."
The Rebate Trap—And How Ironwood Escaped
The U.S. drug pricing system creates a perverse dynamic: the higher a drug's list price, the larger the mandatory rebates owed to government programs. When Congress removed the Medicaid rebate cap in 2024 (effective January 1, 2024), companies suddenly faced rebates that could exceed 100% of a drug's price in some cases.
For LINZESS—the #1 prescribed brand for irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC)—this was a mounting problem. The Inflation Reduction Act's new rebate structure meant Ironwood was hemorrhaging margin on every Medicaid prescription.
The solution? Cut the list price to reset the rebate baseline.
"Effective January 1, 2026, the LINZESS list price has been lowered in response to evolving health care dynamics and to support ongoing patient access," CEO Tom McCourt stated. "In turn, we expect higher net sales in 2026 for LINZESS year-over-year, specifically driven by the elimination of the inflationary component of statutory required rebates across channels, including Medicaid, due to the decrease in list price."
A Template for Big Pharma?
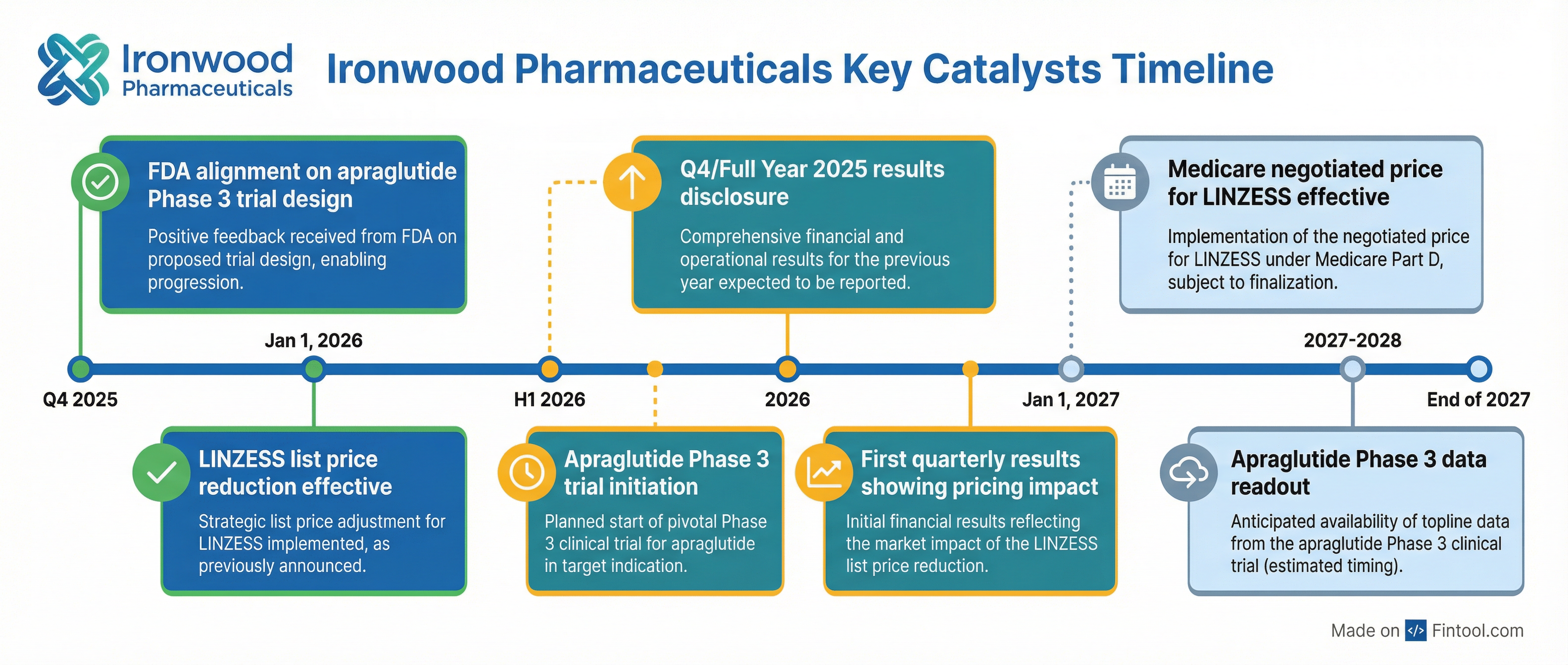
Ironwood may be the first to execute this strategy at scale, but it won't be the last. With the Inflation Reduction Act now requiring Medicare price negotiations for high-expenditure drugs—LINZESS was selected for the second round, with negotiated prices effective January 1, 2027—pharma companies are looking for ways to optimize pricing before government-mandated cuts arrive.
The HHS announced in January 2025 that LINZESS was among 15 drugs selected for Medicare price negotiations, alongside blockbuster treatments for diabetes, chronic kidney disease, and rheumatoid arthritis.
For Ironwood, preemptively cutting list prices achieves multiple objectives:
- Eliminates inflationary rebates across Medicaid and other statutory programs
- Improves net realization per prescription filled
- Supports patient access by reducing out-of-pocket costs
- Positions favorably for Medicare negotiations with lower starting prices
The Abbvie Connection
LINZESS is co-developed and co-commercialized with Abbvie in the United States, with the two companies splitting U.S. brand collaboration profits equally. In Q3 2025, LINZESS U.S. net sales reached $314.9 million, up 40% year-over-year, with net profit for the collaboration hitting $233.1 million—a 67% increase.
The improved pricing dynamics in 2026 will benefit both partners, though AbbVie's massive scale ($58B revenue in 2024) means LINZESS is a rounding error on their income statement. For Ironwood, it's existential.
| Metric | Q3 2024 | Q3 2025 | YoY Change |
|---|---|---|---|
| LINZESS U.S. Net Sales | $225.5M | $314.9M | +40% |
| LINZESS Commercial Margin | 65% | 76% | +11pp |
| Collaboration Net Profit | $139.6M | $233.1M | +67% |
Pipeline Progress: Apraglutide Phase 3 on Track
Beyond LINZESS optimization, Ironwood is advancing apraglutide, a next-generation GLP-2 analog for short bowel syndrome patients dependent on parenteral support. The company met with the FDA in Q4 2025 to align on a confirmatory Phase 3 trial design and expects to initiate the trial in H1 2026.
If approved, apraglutide would be the first and only GLP-2 treatment to achieve statistically significant reduction in weekly parenteral support volume with once-weekly dosing.
The Balance Sheet Question
Despite the upbeat guidance, Ironwood carries meaningful financial risk. The company has approximately $598 million in total debt against $200 million in cash as of Q4 2025. Third-party metrics flag an Altman Z-Score of -3.4, which some interpret as elevated bankruptcy risk.
Management explicitly addressed this: "Throughout 2025, we made significant progress in maximizing LINZESS while delivering sustained profits and cash flows in an effort to strengthen our financial position and maintain compliance with debt covenants over the coming quarters."
| Balance Sheet Metric | Q4 2024 | Q3 2025 | Q4 2025 |
|---|---|---|---|
| Cash & Equivalents | $88.6M | $140.4M | >$200M |
| Total Debt | $599.5M | $598.2M | $598M* |
*Values retrieved from S&P Global
The projected >$300 million in adjusted EBITDA for 2026 would provide substantial deleveraging capacity if the company chooses to pay down debt rather than pursue additional strategic investments.
Strategic Alternatives Still in Play
Ironwood continues its previously announced strategic alternatives review with Goldman Sachs, exploring options to maximize shareholder value. The process began in April 2025 after the FDA required a confirmatory Phase 3 trial for apraglutide, extending the timeline to potential approval.
With LINZESS generating substantial cash flow and a cleaner pricing structure in place, Ironwood could be an attractive acquisition target for larger GI-focused pharma companies or private equity buyers looking for stable, optimizable cash flows.
"We continue to progress our previously announced strategic alternatives review in an effort to maximize shareholder value and look forward to providing further updates as appropriate," McCourt stated.
The Bigger Picture: Drug Pricing Innovation
Ironwood's pricing reset may signal a new era in pharmaceutical economics. As the Inflation Reduction Act reshapes the industry, companies are being forced to think creatively about how to preserve margins while navigating mandatory rebates and government-negotiated prices.
The traditional pharma playbook—annual list price increases to offset volume declines—no longer works when those increases trigger exponentially larger rebates. Ironwood's approach inverts the logic: lower list prices can actually improve net economics when the rebate burden is heavy enough.
For investors, this creates a new screening criterion: which other drugs face similar rebate dynamics, and which companies might benefit from a similar reset?
What to Watch
Near-term catalysts:
- Q4 2025 results and 2026 guidance details (expected later this quarter)
- Apraglutide Phase 3 trial design disclosure
- Updates on strategic alternatives review
2026 milestones:
- Apraglutide Phase 3 initiation (H1 2026)
- First quarterly results showing impact of price reset
- Medicare price negotiation updates for 2027 implementation
Key risks:
- LINZESS demand growth disappointing
- Net pricing improvement less than projected
- Strategic review yields no actionable outcome
- Debt covenant compliance in transition period