Earnings summaries and quarterly performance for AbbVie.
Executive leadership at AbbVie.
Robert A. Michael
Chief Executive Officer
Azita Saleki-Gerhardt
Executive Vice President, Chief Operations Officer
Jeffrey R. Stewart
Executive Vice President, Chief Commercial Officer
Perry C. Siatis
Executive Vice President, General Counsel and Secretary
Scott T. Reents
Executive Vice President, Chief Financial Officer
Board of directors at AbbVie.
Brett J. Hart
Director
Edward J. Rapp
Director
Frederick H. Waddell
Director
Jennifer L. Davis
Director
Melody B. Meyer
Director
Rebecca B. Roberts
Director
Robert J. Alpern
Director
Roxanne S. Austin
Lead Independent Director
Susan E. Quaggin
Director
Thomas C. Freyman
Director
Thomas J. Falk
Director
William H.L. Burnside
Director
Research analysts who have asked questions during AbbVie earnings calls.
Mohit Bansal
Wells Fargo & Company
8 questions for ABBV
Steve Scala
Cowen
8 questions for ABBV
Terence Flynn
Morgan Stanley
8 questions for ABBV
Vamil Divan
Guggenheim Securities
8 questions for ABBV
David Risinger
Leerink Partners
7 questions for ABBV
Christopher Schott
JPMorgan Chase & Co.
6 questions for ABBV
Asad Haider
Goldman Sachs
5 questions for ABBV
Luisa Hector
Berenberg
5 questions for ABBV
Carter L. Gould
Barclays
3 questions for ABBV
Courtney Breen
AllianceBernstein
3 questions for ABBV
David Amsellem
Piper Sandler Companies
3 questions for ABBV
Geoffrey Meacham
Citi
3 questions for ABBV
Trung Huynh
UBS Group AG
3 questions for ABBV
Chris Schott
JPMorgan Chase & Company
2 questions for ABBV
Chris Shibutani
Goldman Sachs Group, Inc.
2 questions for ABBV
Geoff Meacham
Citigroup Inc.
2 questions for ABBV
James Shin
Analyst
2 questions for ABBV
Mark Hoffman
BMO Capital Markets
2 questions for ABBV
Matthew Phipps
William Blair
2 questions for ABBV
Michael Yee
Jefferies
2 questions for ABBV
Simon Baker
Rothschild & Co Redburn
2 questions for ABBV
Timothy Anderson
BofA Securities
2 questions for ABBV
Alexandria Hammond
Wolfe Research
1 question for ABBV
Christopher Raymond
Piper Sandler
1 question for ABBV
David Aslam
Piper Sandler
1 question for ABBV
Evan Seigerman
BMO Capital Markets
1 question for ABBV
Gary Nachman
Raymond James
1 question for ABBV
Jon Win
UBS
1 question for ABBV
Tim Anderson
Bank of America
1 question for ABBV
Recent press releases and 8-K filings for ABBV.
- AbbVie reported risankizumab SC induction met both co-primary endpoints with 55% vs 29.6% clinical remission and 44% vs 14.3% endoscopic response at week 12 (p<0.0001 vs placebo).
- Among patients with clinical response at week 12, 67% achieved clinical remission and 57% achieved endoscopic response at week 24.
- The study enrolled 289 adult patients, predominantly treatment-refractory (65% had failed advanced therapies).
- The safety profile during induction was consistent with known data, with no new safety risks observed.
- On February 24, 2026, AbbVie entered into an underwriting agreement to issue and sell seven series of senior notes totaling $8.0 billion aggregate principal amount due between 2028 and 2066.
- Notes will be priced at or near par, including floating-rate notes due 2028 at 100.000%, 3.775% notes due 2028 at 99.966%, 4.125% notes due 2031 at 99.972%, 4.400% notes due 2033 at 99.861%, 4.750% notes due 2036 at 99.911%, 5.550% notes due 2056 at 99.736% and 5.650% notes due 2066 at 99.714%.
- The offering is expected to close on March 4, 2026, with net proceeds of approximately $7.95 billion.
- AbbVie will use proceeds to repay outstanding borrowings under its $4.0 billion 364-day delayed draw term loan (May 2026 maturity; $2.0 billion currently drawn) and for general corporate purposes, including debt repayment.
- Slate Medicines closed a $130 million Series A financing co-led by RA Capital Management, Forbion, and Foresite Capital.
- Lead program SLTE-1009, licensed from DartsBio, is an anti-PACAP monoclonal antibody designed for subcutaneous preventive migraine dosing, with Phase 1 trials expected mid-2026.
- Slate added Andrew Levin (RA Capital), Tim Lohoff (Forbion), and Cindy Xiong (Foresite) to its Board of Directors as part of the financing.
- The round includes backing from RA Capital, Forbion, Foresite, and an undisclosed biotech investor.
- FDA approval of Calquence plus venetoclax for first-line CLL/SLL, marking the first all-oral, fixed-duration BTK inhibitor–based regimen in the US.
- The 14-month all-oral fixed-duration regimen offers greater flexibility compared with continuous therapies.
- In Phase III AMPLIFY, the 3-year PFS rate was 77% vs 67% for chemoimmunotherapy (HR 0.65; 95% CI, 0.49–0.87; p=0.0038); median PFS was not reached.
- An estimated 18,500 adults received first-line treatment for CLL in the US in 2024, underscoring potential market impact.
- Developed in collaboration with Genentech, the regimen is approved in multiple jurisdictions and is under further global regulatory review.
- The U.S. FDA approved the combination of VENCLEXTA and acalabrutinib as the first all-oral, fixed-duration regimen for previously untreated adult CLL patients.
- Approval is based on the Phase 3 AMPLIFY trial, which showed a 35% reduction in risk of disease progression or death versus chemoimmunotherapy (HR 0.65; p=0.0038), with median progression-free survival not reached versus 47.6 months.
- The regimen is administered over 14 fixed 28-day cycles, offering potential treatment-free intervals and expanding first-line CLL options.
- Safety findings were consistent with known profiles; tumor lysis syndrome incidence was 0.3%, and serious adverse events included COVID-19 (9%) and second primary malignancies (2.7%).
- AbbVie’s bispecific IL-1α/IL-1β inhibitor lutikizumab and RINVOQ in hidradenitis suppurativa are advancing in phase II, with both bio-experienced and biologic-naive cohorts enrolled and double-blind week 16 data expected by year-end.
- The company favors dual IL-1α/β targeting for potential clinical differentiation over IL-1β-only (O09) or OX40-TNF bispecific approaches, citing synergy in blocking key inflammatory pathways and immunogenicity concerns with anti-TNF bispecifics.
- In inflammatory bowel disease, AbbVie is testing combinations of SKYRIZI with a more potent α4β7 antagonist (382) and TL1A blocker (701), while subcutaneous SKYRIZI data and proof-of-concept for a novel TREM1 inhibitor are due later this year.
- A next-generation oral IL-23 program (via Nimble deal) aims to improve potency and extend half-life to match biologic-level efficacy and adherence.
- Early immunology pipeline includes a CD19 ADC (ABBV-319) with steroid payload for rapid B-cell depletion and durable remission, plus a non-payload antibody (ABBV-519) as a potential maintenance therapy.
- AbbVie's bispecific lutikizumab (IL-1α/β) and RINVOQ in hidradenitis suppurativa showed strong Phase II efficacy in TNF-experienced and biologic-naive cohorts; double-blind Week 16 data expected by year-end.
- In IBD, AbbVie is advancing SKYRIZI combination approaches: an α4β7 antagonist plus lutikizumab readout this year and TL1A-SKYRIZI studies starting later this year.
- The TREM1 program (myeloid receptor inhibitor) targets early proof-of-concept data late 2026/early 2027, aiming to improve deep remission in IBD with limited competition in the space.
- AbbVie’s next-gen oral IL-23 asset (via Nimble deal) is designed for higher potency and extended half-life to match biologic efficacy and support adherence.
- A novel 319 ADC targeting CD19 with a steroid payload is being developed for B-cell–driven autoimmunity (e.g., SLE, Sjögren’s), showing deep, durable depletion without systemic steroid effects.
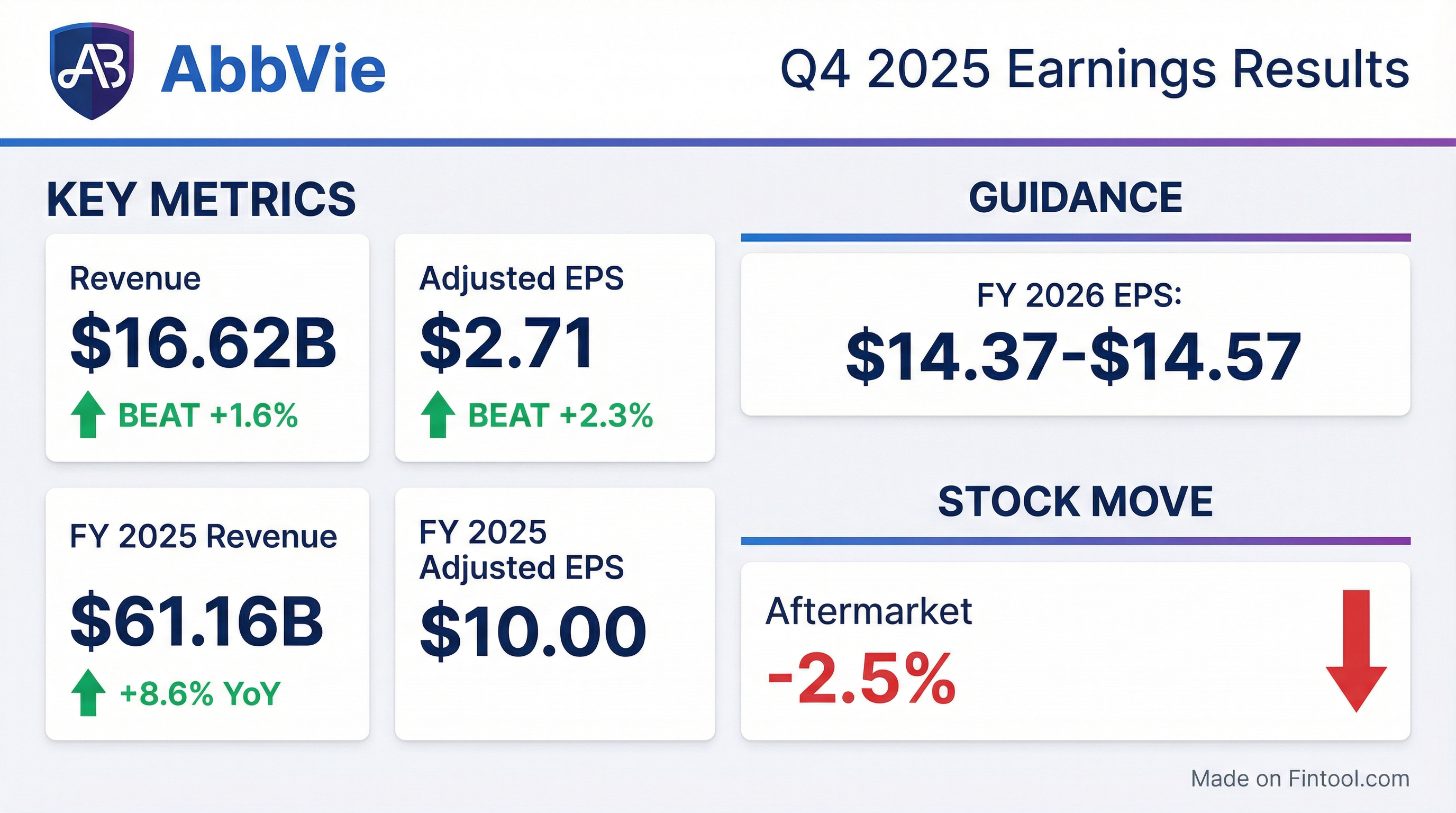
- AbbVie delivered full-year adjusted EPS of $10, beating its guidance midpoint by $0.54 (excluding IPR&D), and achieved record net revenues of $61.2 billion, up 8.6% and $2 billion above guidance despite Humira erosion.
- In Q4 2025, AbbVie reported total net revenues of $16.6 billion, up 10% YoY, and adjusted EPS of $2.71, $0.08 above guidance; the ex-Humira growth platform rose 14.5%, with an adjusted gross margin of 83.6%.
- For 2026, the company forecasts net revenues of ~$67 billion (+9.5%), adjusted EPS of $14.37–14.57, and expects immunology sales of $34.5 billion including Skyrizi at $21.5 billion and Rinvoq at $10.1 billion.
- Immunology performance in Q4 included $8.6 billion in total revenues, with Skyrizi at $5 billion (+31.9%) and Rinvoq at $2.4 billion (+28.6%).
- AbbVie delivered FY 2025 adjusted EPS of $10 and net revenues of $61.2 billion, up 8.6% yoy despite $16 billion of Humira erosion.
- In Q4 2025, AbbVie achieved adjusted EPS of $2.71 and net revenues of $16.6 billion (+10% yoy; ex-Humira platform +14.5%).
- For 2026, AbbVie guides net revenues of ~$67 billion (+9.5% yoy) and adjusted EPS of $14.37–$14.57.
- Immunology franchises delivered Q4 revenues of $8.6 billion, with Skyrizi at $5 billion (+31.9%) and Rinvoq at $2.4 billion (+28.6%); full-year combined revenue reached $25.9 billion (+ > $8 billion yoy).
- AbbVie advanced its pipeline with new approvals (Rinvoq for GCA, Emrelis, Epkinly), funded 90 clinical programs, and invested > $5 billion in business development in 2025.
- Record Q4 net revenues of $16.6 billion and adjusted EPS of $2.71, driving full-year net revenues of $61.2 billion and adjusted EPS of $10.00.
- Skyrizi and Rinvoq combined delivered $25.9 billion in 2025, with Q4 sales of $5 billion and $2.4 billion respectively, while Humira sales declined 26.1% to $1.2 billion in the quarter.
- For 2026, AbbVie guides ~$67 billion in revenues (9.5% growth) and adjusted EPS of $14.37–$14.57, including $34.5 billion in immunology and $12.5 billion in neuroscience sales.
- Continued pipeline investment with $5 billion in business development in 2025 and a three-year US pricing agreement, alongside projected $18.5 billion free cash flow for 2026 to support dividends and BD.
Fintool News
In-depth analysis and coverage of AbbVie.

AbbVie Doubles Down on U.S. Manufacturing with $380 Million API Investment

AbbVie Signs $100 Billion Deal With Trump, Clearing Path for Humira on TrumpRx

AbbVie Pays $650M to Join PD-1/VEGF Bispecific Race in JPM's First Big Deal

AbbVie Nears $20B+ Deal for Revolution Medicines, Capping Historic RAS Rally
Quarterly earnings call transcripts for AbbVie.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more