FDA Reverses Course, Will Review Moderna's mRNA Flu Vaccine
February 18, 2026 · by Fintool Agent

Moderna shares surged 7% Wednesday after the FDA reversed its surprise decision to reject the company's mRNA flu vaccine application—a dramatic about-face that came just eight days after the initial refusal sparked industry-wide alarm about regulatory unpredictability under the new administration.
The FDA has now agreed to review mRNA-1010, Moderna's investigational seasonal influenza vaccine, with a target decision date of August 5, 2026. If approved, the vaccine could be available for adults 50 and older during the fall 2026 flu season.
The Reversal
The FDA's Center for Biologics Evaluation and Research (CBER) had issued a Refusal-to-File letter on February 10, citing concerns about Moderna's use of a licensed standard-dose seasonal flu vaccine as a comparator in its Phase 3 trial. The letter, signed by CBER Director Vinay Prasad, claimed the study did not use a comparator reflecting the "best-available standard of care."
Moderna pushed back immediately, noting the rejection contradicted prior written guidance from the FDA. In April 2024, CBER had explicitly stated that using a standard-dose flu vaccine comparator would be "acceptable" and did not object to the trial protocol before enrollment began.
Following a Type A meeting with regulators, Moderna revised its approach:
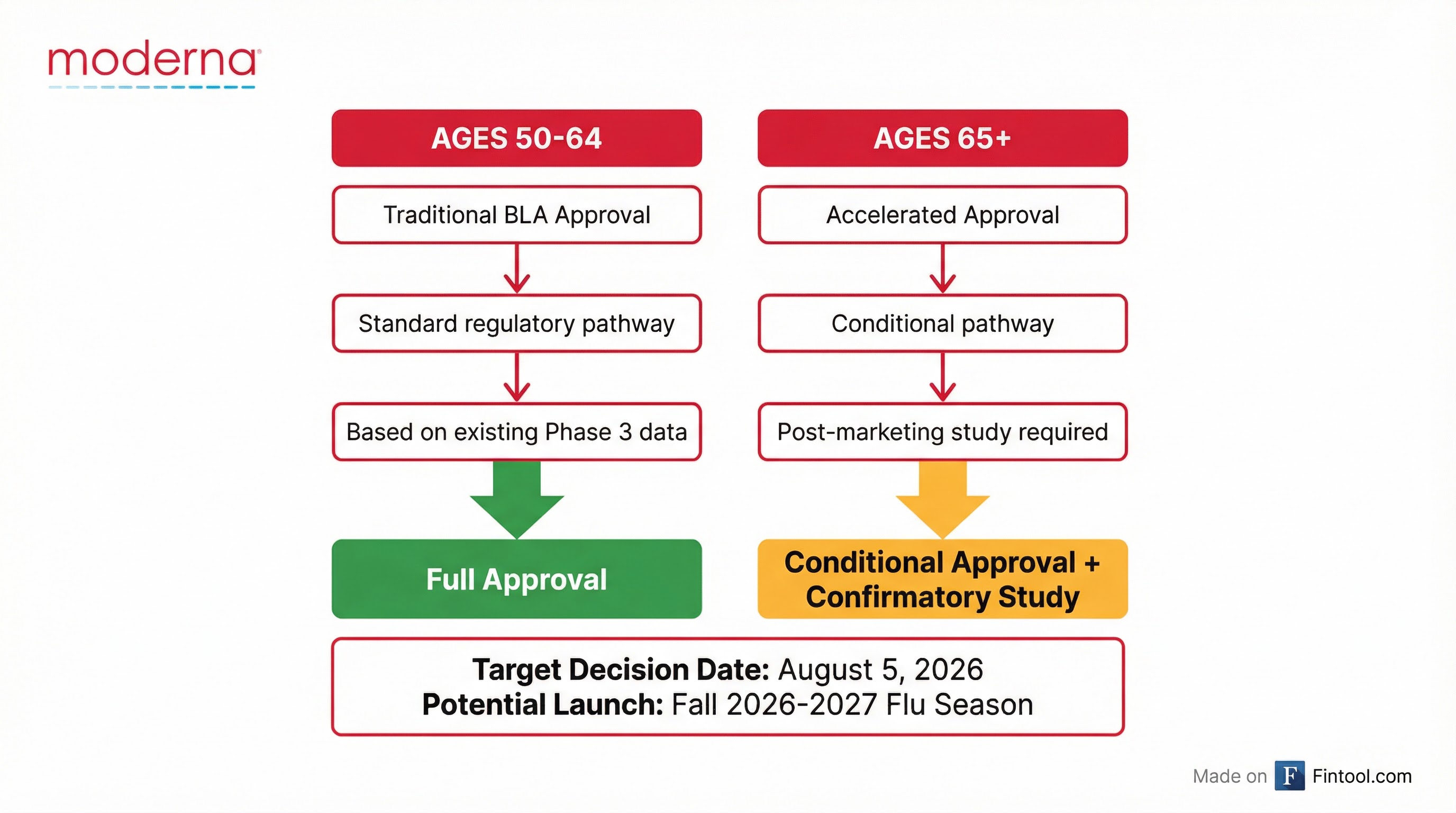
| Pathway | Age Group | Type | Additional Requirements |
|---|---|---|---|
| Traditional BLA | 50-64 years | Full Approval | None |
| Accelerated | 65+ years | Conditional | Post-marketing confirmatory study |
"We appreciate the FDA's engagement in a constructive Type A meeting and its agreement to advance our application for review," CEO Stéphane Bancel said. "Pending FDA approval, we look forward to making our flu vaccine available later this year so that America's seniors have access to a new option to protect themselves against flu."
The Regulatory Timeline
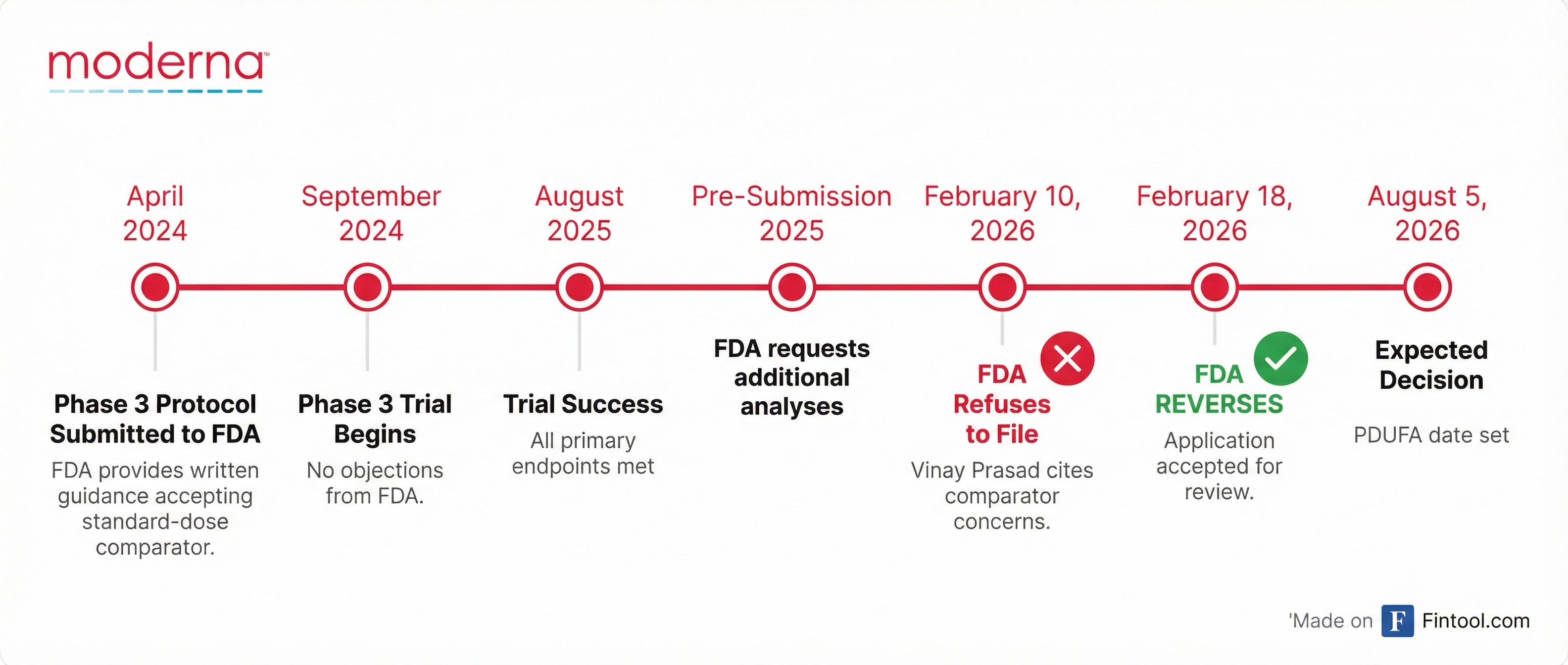
The whiplash from rejection to acceptance in just over a week underscores the chaotic regulatory environment facing vaccine developers. The original refusal was made by Prasad, who STAT News reported had overruled career scientists in the vaccine center.
On Moderna's Q4 2025 earnings call last week, CEO Bancel expressed frustration: "The current uncertainty in the U.S. FDA regulatory environment creates real challenges for businesses, patients, and the broader innovation ecosystem. When expectations and review timelines are unpredictable, companies face greater risk and can hesitate to invest, slowing the development of breakthrough medicines."
Market Reaction
Moderna stock jumped 7% to $47.02 on the news, recovering from a punishing selloff following the initial rejection. The stock had been trading near 52-week lows, with shares down significantly from the 2021-2022 COVID vaccine peak.
| Metric | Value |
|---|---|
| Current Price | $47.02 |
| Day Change | +7.0% |
| 52-Week Range | $22.28 - $55.20 |
| Market Cap | $18.4B |
| Cash Position (Q4 2025) | $2.6B |
The company reported Q4 2025 revenue of $645 million and a net loss of $826 million , as it continues to invest heavily in diversifying beyond COVID vaccines.
Broader Context: mRNA Under Fire
The reversal comes amid broader pressure on mRNA technology under Health Secretary Robert F. Kennedy Jr., who has repeatedly criticized mRNA vaccines as unsafe despite their widespread success during the COVID pandemic.
The Trump administration has canceled hundreds of millions of dollars in mRNA research contracts and sharply limited recommendations for COVID vaccine administration—moves that have sent shockwaves through the vaccine industry.
Moderna's flu vaccine would be the first mRNA flu shot approved in the United States, representing a significant test case for whether the technology can expand beyond COVID. The company notes mRNA-1010 met all pre-specified primary endpoints in its Phase 3 trial and has been accepted for review in the EU, Canada, and Australia with potential approvals expected in late 2026 or early 2027.
What to Watch
August 5, 2026: FDA target decision date for mRNA-1010
Fall 2026: Potential U.S. launch for 2026-2027 flu season if approved
Post-marketing study: Required for accelerated approval in adults 65+
International approvals: EU, Canada, and Australia reviews ongoing
The eight-day turnaround from rejection to acceptance may ease immediate concerns about FDA dysfunction, but questions remain about how the agency will handle future mRNA submissions under the current administration.
Related Companies: Moderna (mrna)