Pharming Guides to $405M-$425M Revenue at 2026 Investor Day, Eyes Two Billion-Dollar Pipeline Programs
February 3, 2026 · by Fintool Agent

Pharming Group used its 2026 Investor Day to unveil bullish financial guidance and showcase two late-stage pipeline programs it believes each carry billion-dollar potential—but the rare disease biotech still faces headwinds from a fresh FDA rejection that has hammered its stock price.
Shares of Pharming (NASDAQ: PHAR) traded at $16.91, down 19% from the $20.69 close on January 30—the day before the FDA issued a Complete Response Letter rejecting the company's bid to expand Joenja (leniolisib) to younger pediatric patients.
2026 Financial Guidance Tops Street Estimates
At its virtual Investor Day, Pharming laid out 2026 revenue guidance of $405 million to $425 million, implying 8% to 13% growth over preliminary 2025 revenues.
That guidance compares favorably to analyst consensus of approximately $398 million, according to CFO Kenneth Lynard.
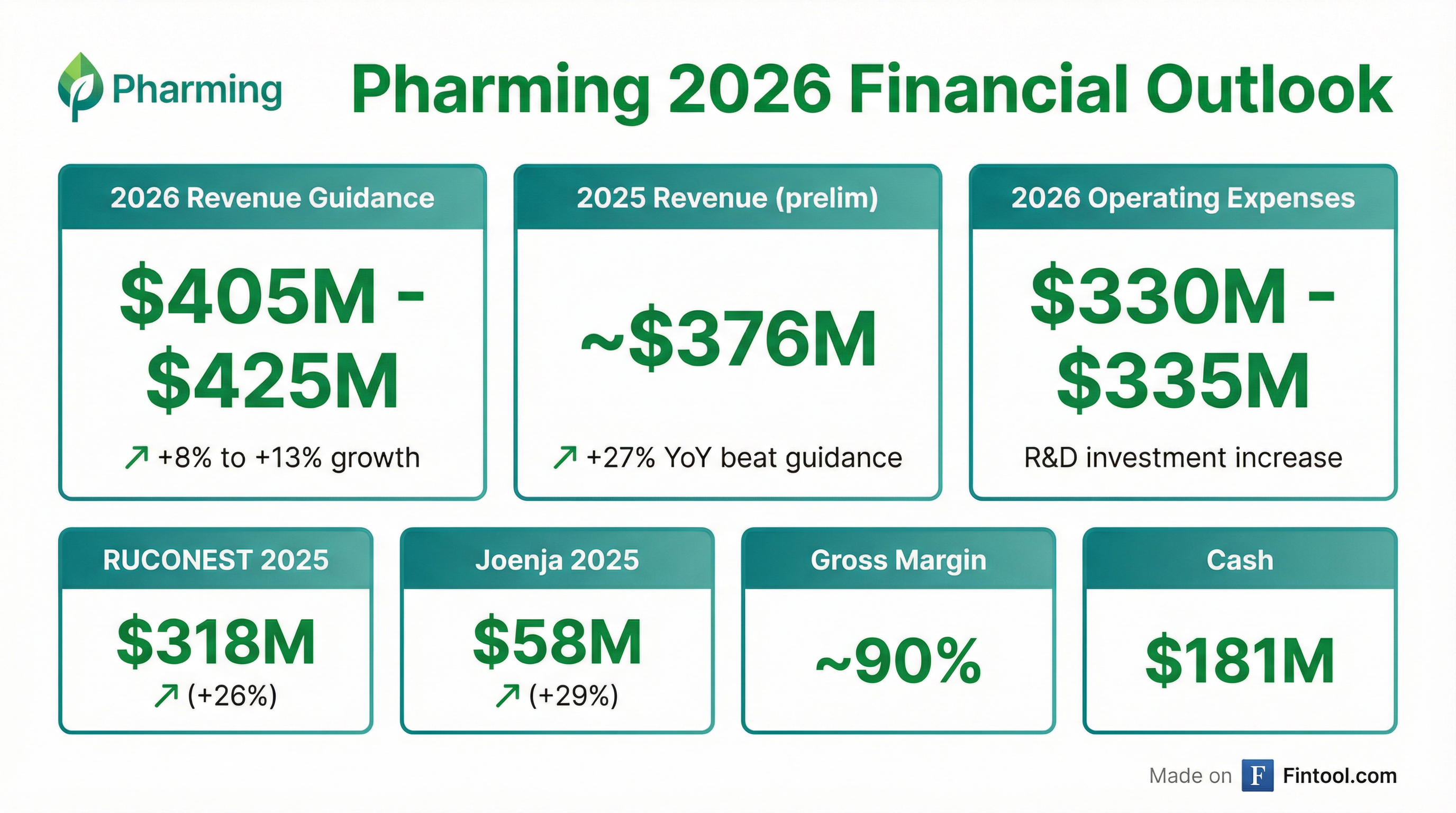
The company reported preliminary 2025 revenue of approximately $376 million—a 27% increase over 2024 that slightly exceeded its upwardly revised guidance of $365 million to $375 million provided in November.
| Metric | FY 2024 | FY 2025 (Prelim) | FY 2026 Guidance |
|---|---|---|---|
| Total Revenue | $297M* | $376M | $405M-$425M |
| RUCONEST Revenue | N/A | $318M | Mid-single-digit growth |
| Joenja Revenue | N/A | $58M | 10pp acceleration |
| Operating Expenses | $304M-$308M | Within guidance | $330M-$335M |
| Gross Margin | 88-89% | 88-89% | 90% |
*Values retrieved from S&P Global
FDA Setback Overshadows Growth Story
The upbeat guidance came just two days after the FDA issued a Complete Response Letter for Pharming's supplemental New Drug Application seeking to expand Joenja to children ages 4 to 11 with activated phosphoinositide 3-kinase delta syndrome (APDS).
The FDA raised concerns about potential underexposure in lower-weight pediatric patients and requested additional pharmacokinetic data for the 20-milligram and 30-milligram doses that apply to children weighing less than 27 kilograms.
"We have a wealth of data at hand," CEO Fabrice Chouraqui told analysts. "There is a possibility that the data that we have will satisfy the FDA. We'll be able to update you on the course of action after the Type A meeting takes place."
That meeting is expected in March.
Importantly, Pharming has conservatively removed any pediatric revenues from its 2026 Joenja guidance, and the current FDA approval for patients 12 and older remains unaffected.
Two Billion-Dollar Pipeline Opportunities
The heart of Pharming's Investor Day focused on pipeline programs the company believes could transform it into "a leading global rare disease company."
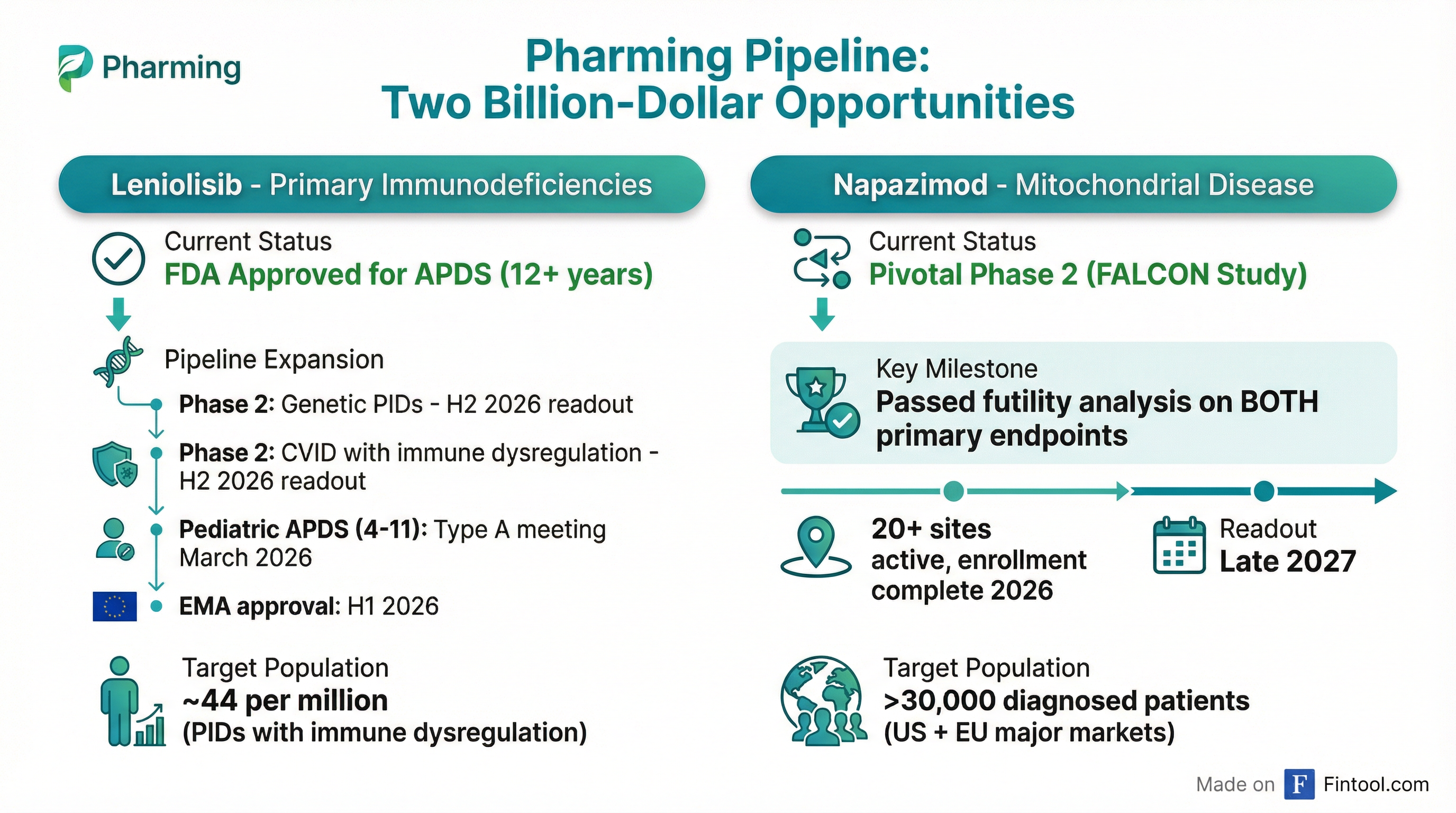
Leniolisib: Expanding Beyond APDS
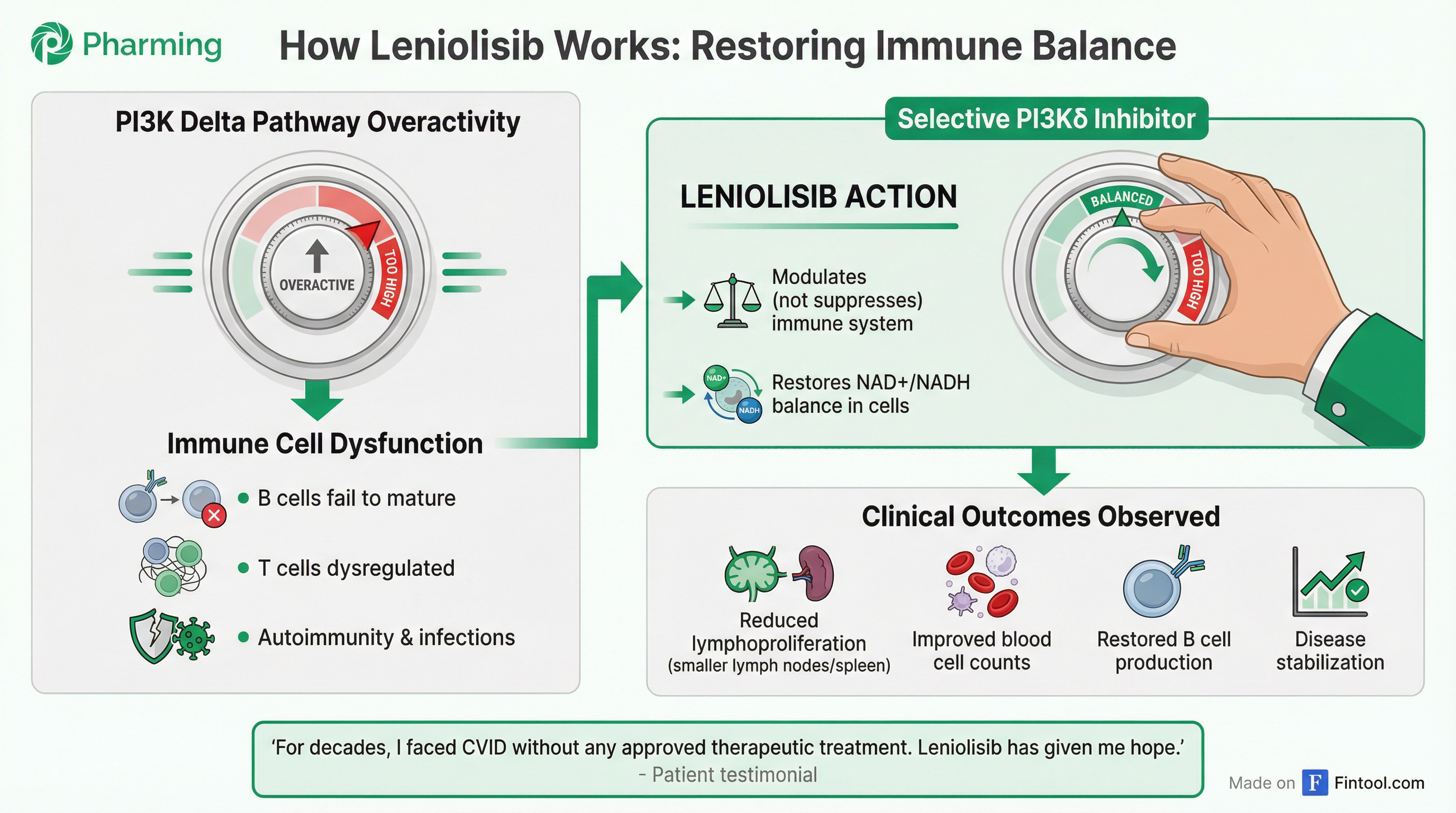
Pharming is running two Phase 2 proof-of-concept trials to expand leniolisib into broader primary immunodeficiency populations—a market the company estimates at 44 patients per million, far larger than the ultra-rare APDS population of 1-2 per million.
Dr. Jocelyn Farmer, Director of the Clinical Immunodeficiency Program at Beth Israel Lahey Health, presented compelling data showing that approximately 75% of patients with common variable immunodeficiency (CVID) share an "APDS-like endotype" that could make them candidates for leniolisib.
"There are no FDA-approved therapies" for the immune dysregulation complications of CVID, Dr. Farmer emphasized. "This is really fresh snow when you think about a therapeutic space."
The company presented compassionate use data from a 64-year-old CVID patient who had failed multiple prior therapies. After one year on leniolisib, she showed stabilized liver disease, doubled T-cell counts, and cleared long COVID.
"For decades, I faced the systemic complexities of CVID without the benefit of any approved therapeutic treatment," the patient wrote. "Leniolisib has given me hope that my immunological deficits can be stabilized and ultimately repaired."
Top-line data from both Phase 2 trials is expected in the second half of 2026.
Napazimod: First Standard of Care for Mitochondrial Disease
Pharming formally introduced "napazimod" as the compound name for KL1333, its candidate for primary mitochondrial disease—a rare metabolic disorder affecting over 30,000 diagnosed patients in the U.S. and major European markets.
The pivotal Phase 2 FALCON study has passed its futility analysis on both primary endpoints—fatigue (PROMIS score) and muscle function (30-Second Sit-to-Stand)—giving management confidence in the program's trajectory.
"We are therefore very confident that as this study continues... we will have a really strong data set," said Dr. Magnus Hansson, Medical Director leading the program.
Dr. Amel Karaa, Director of the Mitochondrial Disease Program at Massachusetts General Hospital, described the devastating impact of the disease: "Most of our patients do have similar disease burden and have a lot of morbidity and mortality... It takes all your quality of life away."
Key milestones for napazimod:
- 20+ sites actively enrolling, with additional 20+ being opened
- Enrollment completion expected by end of 2026
- Pivotal readout expected late 2027
- Open-label extension opening Q2 2026
"We are positioned to become the first standard of care in mitochondrial mtDNA-related disease," Pharming stated.
Commercial Portfolio: RUCONEST Holds, Joenja Accelerates
Despite new oral competition in the hereditary angioedema market from Kalvista, Pharming expressed confidence in RUCONEST's durability.
"RUCONEST is not a treatment that you take by choice. It's a treatment that you take because you need," management explained. "The majority of patients who are treated with RUCONEST have already failed other on-demand treatments. If they are actually well controlled on RUCONEST, they often really want to stay on this drug."
RUCONEST generated $318 million in 2025 revenue (+26% YoY) and is positioned for mid-single-digit growth in 2026, with a 3% price increase in line with CPI.
Joenja continues its acceleration, reaching $58 million in 2025 (+29% YoY) and triggering the first $5 million milestone payment to Novartis. The company expects Joenja growth to accelerate by approximately 10 percentage points in 2026.
Additional Joenja catalysts include:
- EMA approval expected H1 2026
- Japan regulatory review on track for 2026 approval
- VUS reclassification potential (~250 patients) as genetic testing improves
- Higher APDS prevalence data published in Cell suggests larger addressable market
What to Watch
| Catalyst | Timeline | Significance |
|---|---|---|
| Type A FDA Meeting (Pediatric Joenja) | March 2026 | Path forward for pediatric APDS |
| EMA Approval (Leniolisib) | H1 2026 | European market expansion |
| Phase 2 Readouts (PIDs/CVID) | H2 2026 | Billion-dollar market validation |
| FALCON Enrollment Complete | End 2026 | De-risks pivotal trial |
| Q4/FY 2025 Earnings | March 12, 2026 | Final 2025 financials |
| Napazimod Pivotal Readout | Late 2027 | First-in-class approval potential |
Pharming ended 2025 with $181 million in cash and marketable securities and expects available cash and future cash flows to cover pipeline investments and pre-launch costs.
Related Research:
- Pharming Group Company Profile
- Novartis Company Profile (Joenja milestone partner)