Shionogi Bets $2.5 Billion on ALS Drug RADICAVA, Building Rare Disease Platform
December 22, 2025 · by Fintool Agent

Japanese pharmaceutical company Shionogi & Co. has agreed to acquire global rights to RADICAVA, one of only a handful of FDA-approved treatments for amyotrophic lateral sclerosis (ALS), from Tanabe Pharma Corporation for $2.5 billion in cash. The deal, announced today, will add approximately $700 million in annual revenue and establishes Shionogi's first major commercial presence in the rare disease market.
For the roughly 30,000 Americans living with ALS—a progressive, fatal neurodegenerative disease with no cure—RADICAVA represents one of the few therapeutic options that can slow functional decline. The transaction positions Shionogi to not only steward this critical medicine but also leverage the acquired infrastructure to launch future rare disease therapies currently in its pipeline.
The Deal
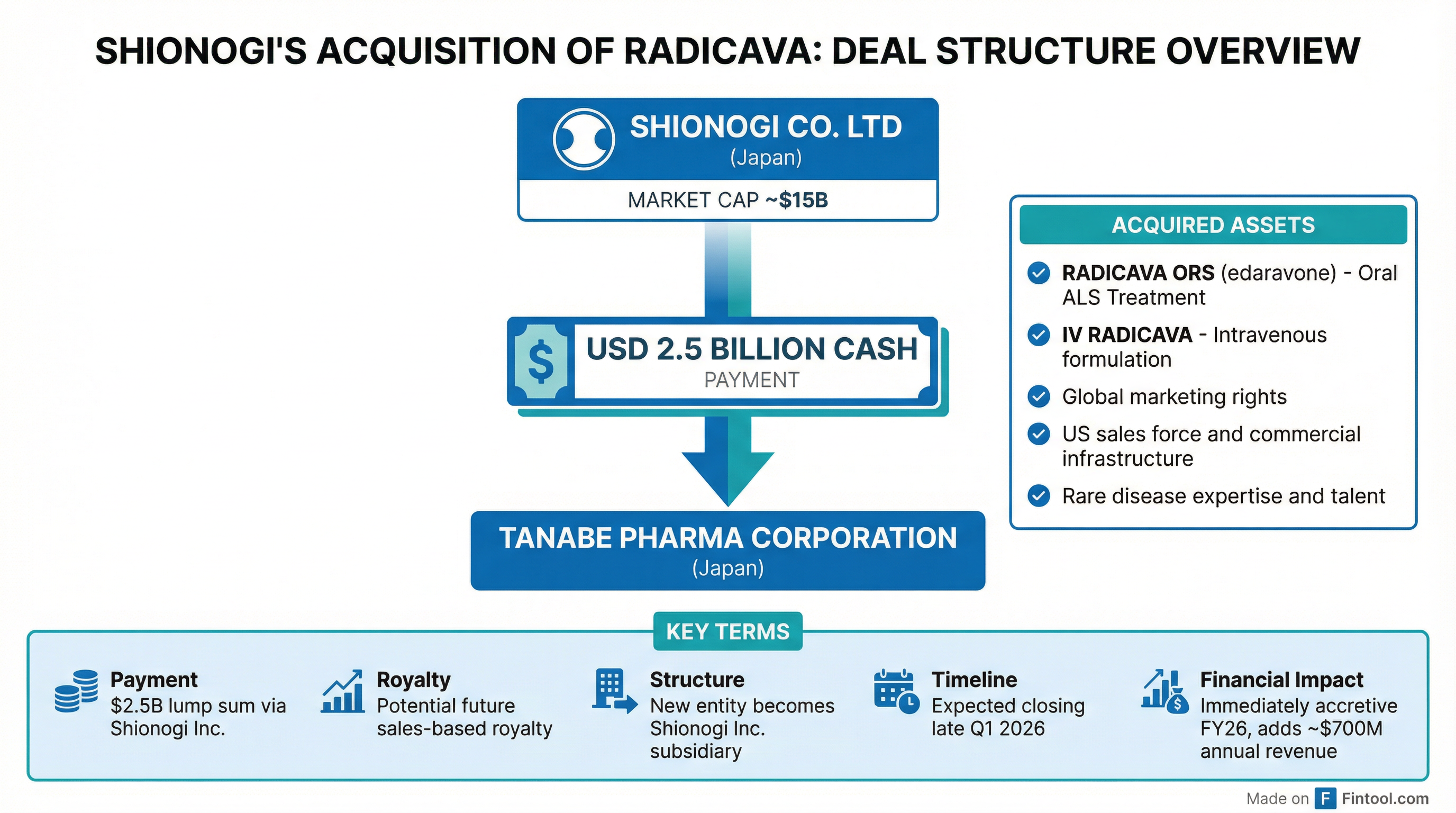
Under the agreement, Shionogi will pay $2.5 billion to Tanabe Pharma through its U.S. subsidiary, Shionogi Inc. Tanabe will create a new entity to hold the RADICAVA business, which Shionogi will acquire in its entirety. The new company will become a wholly owned subsidiary of Shionogi Inc. upon closing, expected in late Q1 2026.
The acquisition includes:
- RADICAVA ORS (edaravone) – The oral suspension formulation approved by the FDA in May 2022, which has become the preferred administration method for patients
- IV RADICAVA – The original intravenous formulation approved in May 2017
- Global marketing rights – Including the U.S., Canada, Switzerland, Australia, Brazil, and other markets where the drug is approved
- Commercial infrastructure – The established U.S. sales force and rare disease commercial team
- Regulatory expertise – Orphan drug designation and related exclusivity protections
Shionogi may also pay future royalties on sales, subject to certain conditions, though specific terms were not disclosed.
"We are pleased to welcome RADICAVA to Shionogi. This critically important medicine was created to reduce the devastating burden of ALS, and we are honored to assume responsibility for it," said Isao Teshirogi, Ph.D., CEO of Shionogi. "We recognize the significant responsibility that comes with a widely utilized breakthrough medicine and will be conscientious stewards of this important drug."
What Shionogi Is Buying
RADICAVA (edaravone) is a free radical scavenger that reduces oxidative stress in motor neurons. While the precise mechanism in ALS is not fully understood, clinical trials demonstrated that treatment slowed the loss of physical function by approximately 33% compared to placebo over 24 weeks, as measured by the ALS Functional Rating Scale-Revised (ALSFRS-R).
| Metric | Details |
|---|---|
| FDA Approvals | IV (May 2017), Oral (May 2022) |
| Patients Treated in U.S. | 20,000 |
| Annual Global Sales | $700 million |
| Administration | Daily for 14 days on, 14 days off (initial cycle) |
| Wholesale Acquisition Cost | $12,719 per 28-day supply (oral) |
| Orphan Drug Exclusivity | Granted 2024 for oral formulation |
The oral formulation, RADICAVA ORS, was a significant advancement for patient convenience. ALS patients often experience progressive mobility limitations, making intravenous infusions increasingly burdensome. The oral suspension can be self-administered at home, reducing the treatment burden for patients and caregivers.
In 2024, the FDA granted RADICAVA ORS Orphan Drug Exclusivity based on its "major contribution to patient care" by providing an oral route of administration that avoids the burdens of IV infusion. This designation provides market exclusivity protection and underscores the drug's importance to the ALS patient community.
The ALS Treatment Landscape
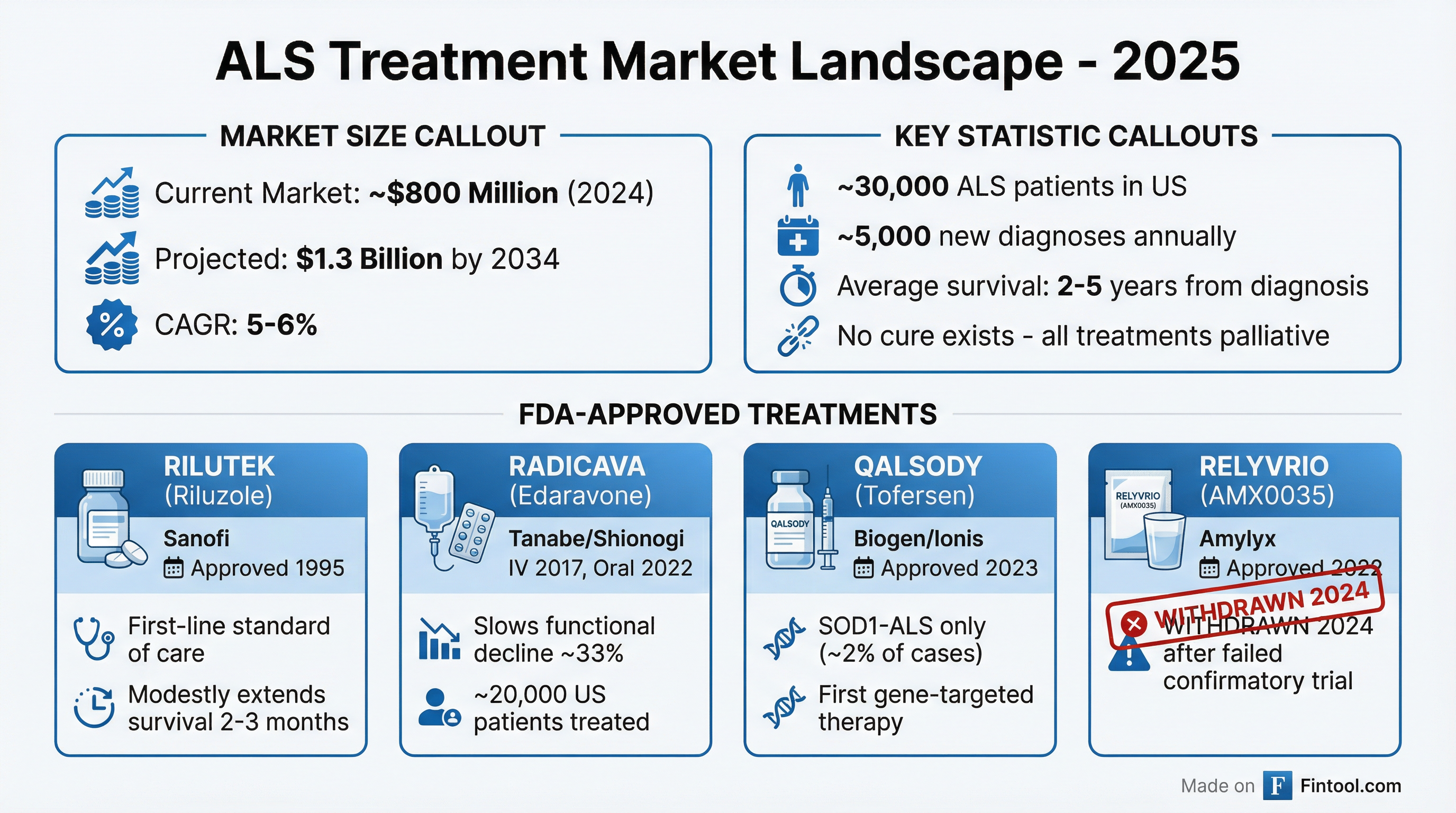
Amyotrophic lateral sclerosis remains one of medicine's most challenging diseases. Patients progressively lose the ability to move, speak, swallow, and eventually breathe, with median survival of only 2-5 years from diagnosis. There is no cure, and treatment options remain limited.
The ALS drug market, currently valued at approximately $800 million, is expected to grow to $1.3 billion by 2034. Yet despite billions invested in research, only a handful of FDA-approved treatments exist:
RILUTEK (riluzole) – Approved in 1995, this glutamate inhibitor remains the first-line standard of care, modestly extending survival by 2-3 months. Developed by Sanofi, it is now available as a generic.
RADICAVA (edaravone) – The second major ALS drug, now belonging to Shionogi, which has treated over 20,000 patients in the U.S. since its approval.
QALSODY (tofersen) – Approved in April 2023 by Biogen+8.53% and Ionis Pharmaceuticals, this antisense oligonucleotide targets the genetic cause of SOD1-ALS. However, this form accounts for only about 2% of all ALS cases, limiting the addressable patient population.
RELYVRIO (AMX0035) – Amylyx Pharmaceuticals received FDA approval in September 2022, but the drug was withdrawn from the market in 2024 after a confirmatory trial failed to demonstrate clinical benefit—a stark reminder of the challenges in ALS drug development.
The withdrawal of RELYVRIO leaves RADICAVA as the leading non-generic treatment option for the broader ALS population, enhancing its strategic value. With limited competition and significant unmet medical need, the acquisition appears well-timed.
Strategic Rationale
For Shionogi, this acquisition is about more than a single drug. The deal creates the commercial infrastructure to accelerate delivery of future rare disease therapies—a strategic priority the company has been building toward.
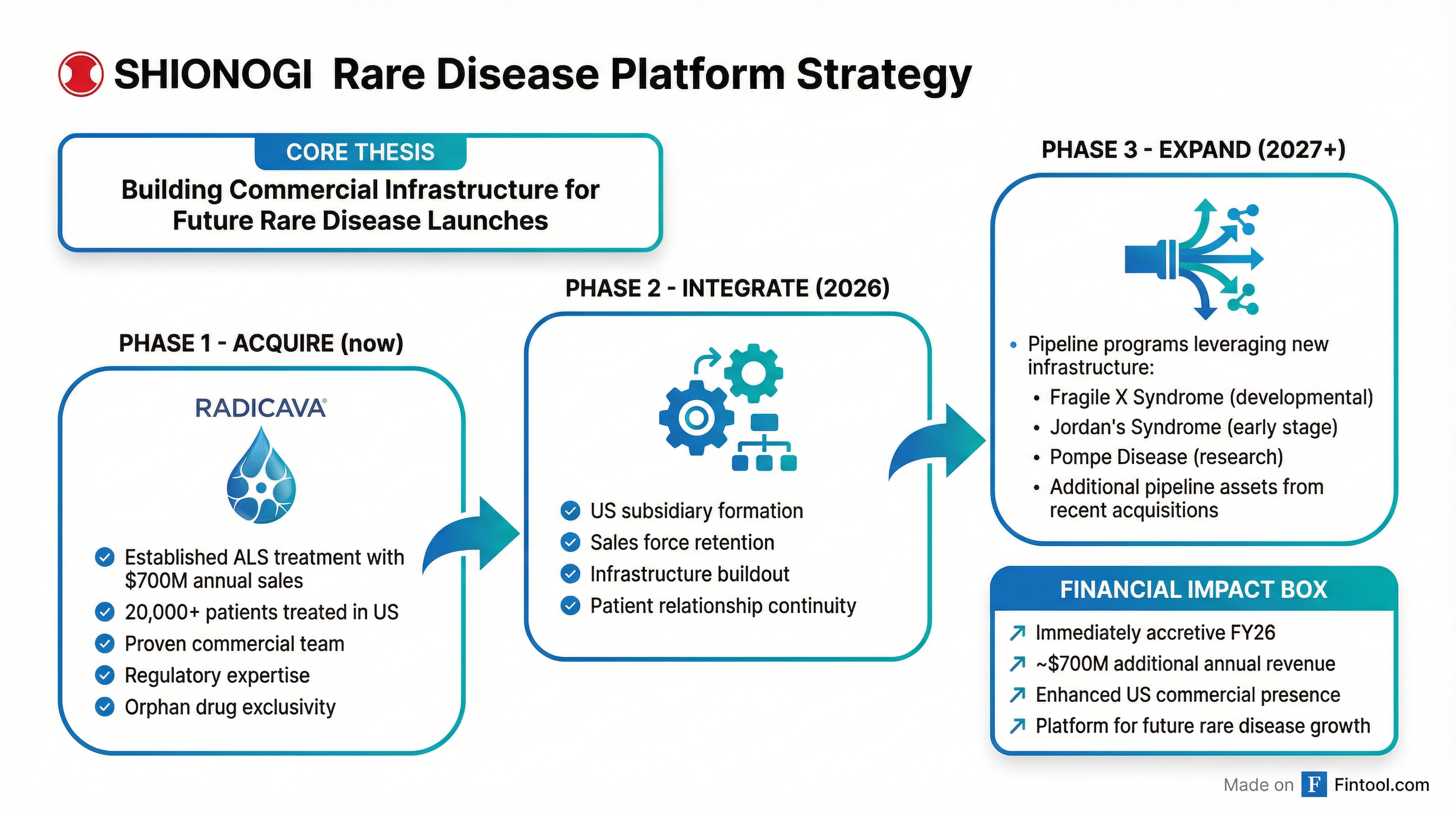
"This acquisition will solidify our strategic focus in rare disease and immediately add capabilities to ensure long-term success in this important category," said Nathan McCutcheon, President and CEO of Shionogi Inc. "Going forward, this infrastructure will support future launches in rare disease including Shionogi's development programs in Fragile X syndrome, Jordan's Syndrome, and Pompe disease."
Shionogi has identified rare diseases as a priority within its broader focus on "high social impact QOL disorders." The company's pipeline includes multiple early-stage programs that could leverage the acquired commercial platform:
| Program | Indication | Stage |
|---|---|---|
| Fragile X Syndrome | Rare genetic disorder causing intellectual disability | Research |
| Jordan's Syndrome | Ultra-rare neurodevelopmental disorder | Early Development |
| Pompe Disease | Rare metabolic disorder affecting muscles | Research |
| Additional assets | From recent pipeline acquisitions | Various |
The financial profile is attractive. Shionogi, with a market capitalization of approximately $15 billion, expects the acquisition to be immediately accretive in fiscal year 2026. The $700 million in annual sales represents a meaningful addition to Shionogi's revenue base, which was approximately $2.9 billion in fiscal year 2024.
What to Watch
Patient continuity: Shionogi has emphasized that safe transition of the medicine and patient relationships is the top priority. The company plans to retain RADICAVA's experienced commercial team to maintain continuity of care for the thousands of patients currently on therapy.
Pipeline leverage: Investors should monitor whether Shionogi successfully leverages the acquired infrastructure to advance its rare disease pipeline. The strategic value of the deal depends significantly on future launches benefiting from the platform.
ALS drug development: The broader ALS landscape remains active. Multiple companies are pursuing new mechanisms of action, including gene therapies and targeted antisense approaches. Shionogi's position with RADICAVA provides a commercial beachhead but also exposure to potential competitive entrants.
Regulatory trajectory: RADICAVA ORS's Orphan Drug Exclusivity provides market protection, but the competitive dynamics could shift if new treatments prove more effective or convenient. The FDA's accelerated approval pathway continues to evolve, particularly following the RELYVRIO withdrawal.
Integration execution: Large cross-border pharmaceutical acquisitions carry integration risk. Shionogi's ability to retain key talent and maintain commercial momentum during the transition will be critical.
Shionogi & Co., Ltd. trades on the Tokyo Stock Exchange (4507.T) and as an ADR in the U.S. (Sgioy+0.18%). Tanabe Pharma Corporation is a subsidiary of Mitsubishi Chemical Group. Related companies mentioned include Biogen+8.53% (QALSODY for SOD1-ALS).
Photo: Fintool