TG Therapeutics Eyes $900M Revenue as BRIUMVI Takes Market Share from Roche, Novartis
January 13, 2026 · by Fintool Agent

TG Therapeutics shares rose 6.7% after hours Tuesday after the company raised its 2026 revenue guidance to $875-900 million at the J.P. Morgan Healthcare Conference—a 42% increase over 2025's preliminary $616 million. The biotech's flagship multiple sclerosis drug BRIUMVI continues to capture market share from established players Roche's Ocrevus and Novartis's Kesimpta in the $10 billion anti-CD20 MS treatment market.
"BRIUMVI continued to deliver strong commercial performance in 2025, reinforcing our confidence in the multi-billion dollar opportunity for BRIUMVI," CEO Michael Weiss said. "With significant market share capture since launch, we believe TG Therapeutics is well positioned to drive long-term revenue growth and cash flow."
From Zero to $900 Million in Three Years
The numbers tell a remarkable story. BRIUMVI received FDA approval in December 2022, and TG Therapeutics has since executed one of biotech's most successful drug launches in recent memory.
| Period | Revenue | Growth |
|---|---|---|
| Q4 2023 | $44M | -- |
| Q4 2024 | $108M | +146% YoY |
| Q3 2025 | $162M | +93% YoY |
| Q4 2025E | $182M | +69% YoY |
| FY 2025 | $616M | -- |
| FY 2026E | $875-900M | +42% YoY |
Quarterly revenue has grown sequentially every quarter since launch. The company's preliminary Q4 2025 BRIUMVI U.S. net product revenue of approximately $182 million implies full-year U.S. sales of $594 million, with international sales contributing the remaining $22 million through partner Neuraxpharm.
The Anti-CD20 Battleground
BRIUMVI competes in the anti-CD20 monoclonal antibody class, where it faces two established competitors: Roche's Ocrevus and Novartis's Kesimpta.
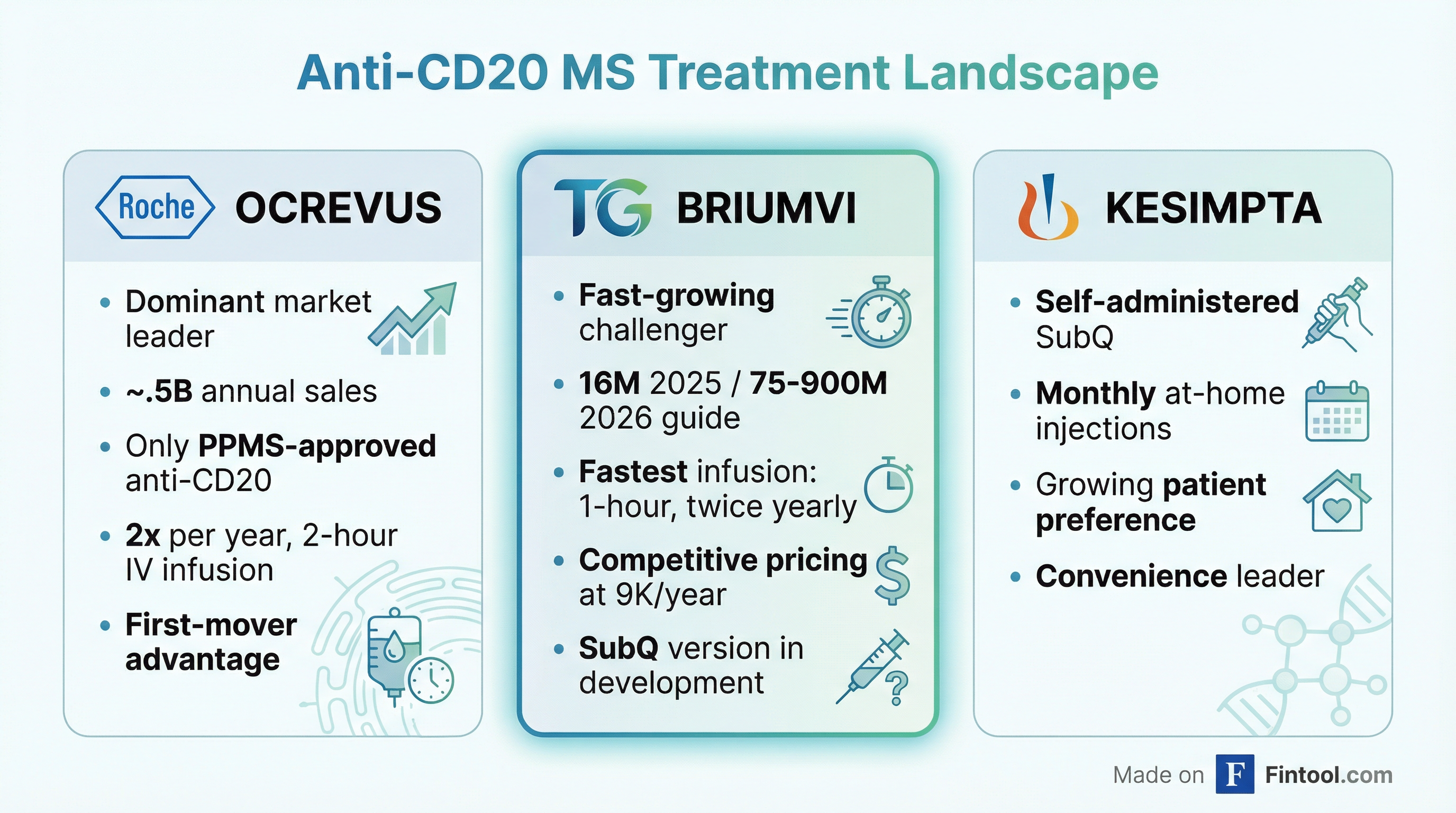
The anti-CD20 class now represents nearly $10 billion in annual U.S. MS sales, yet approximately half of all MS patients remain on other types of disease-modifying therapies—underscoring the significant runway for continued growth.
BRIUMVI's differentiation centers on three factors:
Speed. BRIUMVI requires just a one-hour infusion twice yearly—the fastest in the class. Ocrevus requires two-hour infusions.
Efficacy. Six-year data from the ULTIMATE trials show nearly 90% of patients remain free from disability progression, with an annualized relapse rate of just 0.012—equivalent to one relapse every 83 years of patient treatment.
Price. BRIUMVI is competitively priced at approximately $59,000 annually, positioning it as the most affordable anti-CD20 MS treatment in the U.S.
The market is currently split roughly 65/35 between IV infusions and subcutaneous self-administration. Kesimpta's at-home convenience has driven its growth in the subcu segment, which is why TG is developing its own subcutaneous BRIUMVI—a product that management believes could nearly double the addressable market.
Pipeline Catalysts Stack Up Through 2028
TG outlined multiple development milestones for 2026 and beyond:
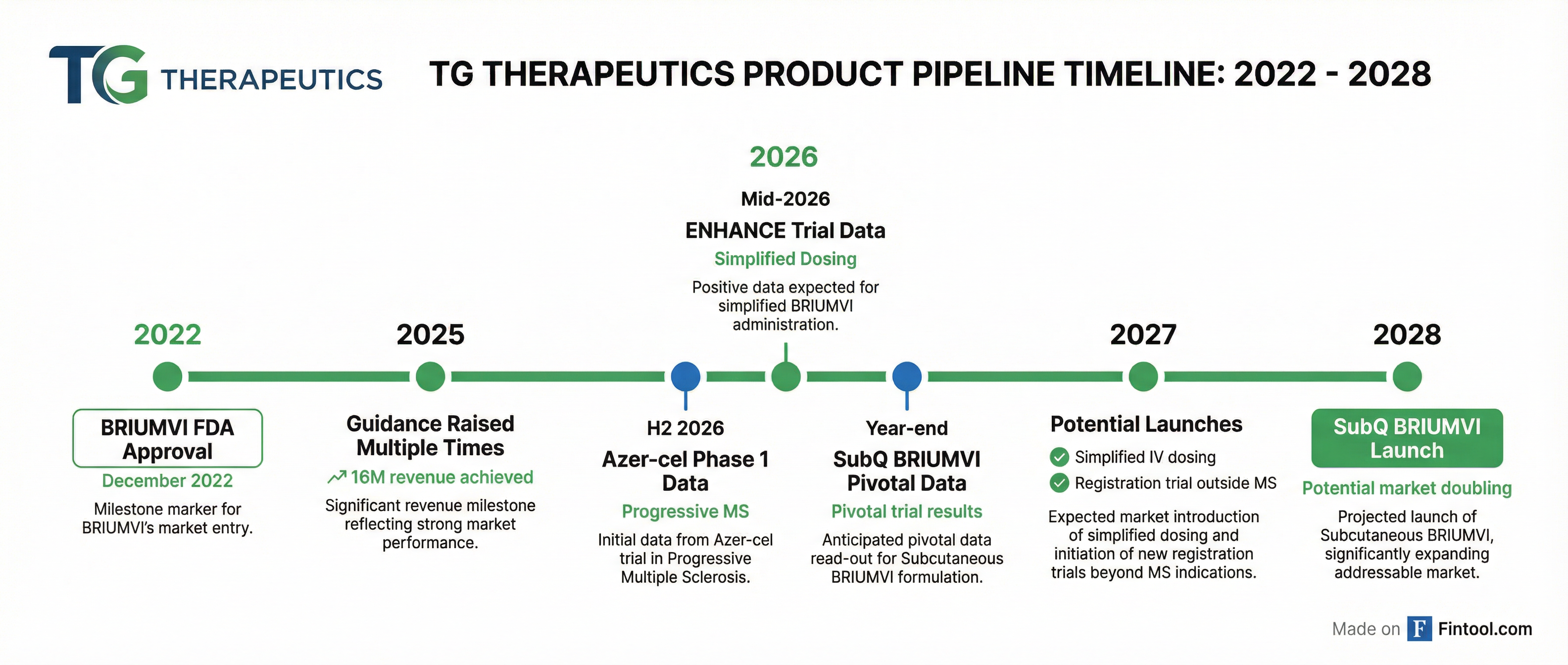
Mid-2026: ENHANCE Trial Data. This Phase 3 pivotal study explores whether the Day 1 and Day 15 doses of IV BRIUMVI can be consolidated into a single Day 1 infusion while maintaining bioequivalent exposure. Enrollment completed faster than expected, signaling strong physician interest. If positive, a simplified dosing schedule could launch in 2027.
H2 2026: Azer-cel Phase 1 Data. TG is developing an allogeneic CAR-T therapy for patients with progressive MS—a population with limited treatment options. "For people living with progressive MS, this type of therapy could be life-changing," Weiss said.
Year-end 2026/Q1 2027: Subcutaneous BRIUMVI Pivotal Data. The Phase 3 trial tests two dosing schedules—once every other month and once quarterly—for a self-administered product. If approved, TG would be the only company offering both IV and self-administered CD20 options for MS.
2026: Registration Trial Outside MS. The company plans to commence a registration-directed trial for BRIUMVI in an indication beyond MS, potentially positioning BRIUMVI as a "pipeline-in-a-product." Management has treated a small number of myasthenia gravis patients with encouraging results.
Financial Discipline Amid Growth
TG expects 2026 operating expenses of approximately $350 million (excluding non-cash compensation), plus about $100 million for subcutaneous BRIUMVI inventory build and secondary manufacturer startup costs.
The company has been profitable for six consecutive quarters. Q3 2025 GAAP net income was $390.9 million, though this included a non-recurring $365 million tax benefit from releasing the deferred tax asset valuation allowance.
| Metric | Q3 2025 | Q2 2025 | Q1 2025 | Q4 2024 |
|---|---|---|---|---|
| Revenue | $162M | $141M | $121M | $108M |
| Gross Margin | 82.6%* | 86.6%* | 87.1%* | 85.8%* |
| Diluted EPS | $2.43 | $0.17 | $0.03 | $0.15 |
*Values retrieved from S&P Global
The company completed a $100 million share repurchase program during 2025, buying back approximately 3.5 million shares at an average price of $28.55. The board has authorized another $100 million program.
"When we can't deploy capital better inside the business, we return it to shareholders," Weiss said. On M&A, he added: "We've seen every announced deal... we have a high standard for ROI. We have a lot of good things in the portfolio already."
What to Watch
Q4 2025 earnings. TG will report final 2025 results and provide more detail on 2026 expectations. Watch for persistence metrics—patient retention has exceeded expectations and is becoming an increasingly larger part of the business.
ENHANCE topline data. Mid-2026 readout could simplify treatment and drive additional market share.
Subcutaneous filing timeline. Pivotal data by early 2027 could set up a 2028 launch, potentially doubling the addressable market.
DTC campaign impact. TG's national television campaign launched in Q3 2025. Early indicators—branded search activity, website traffic, and brand awareness—are "encouraging" but remain to be seen in conversion rates.
Related Companies: TG Therapeutics | Roche | Novartis