Viatris Taps M&A Veteran to Replace 20-Year CLO Amid Legal Storm
February 3, 2026 · by Fintool Agent

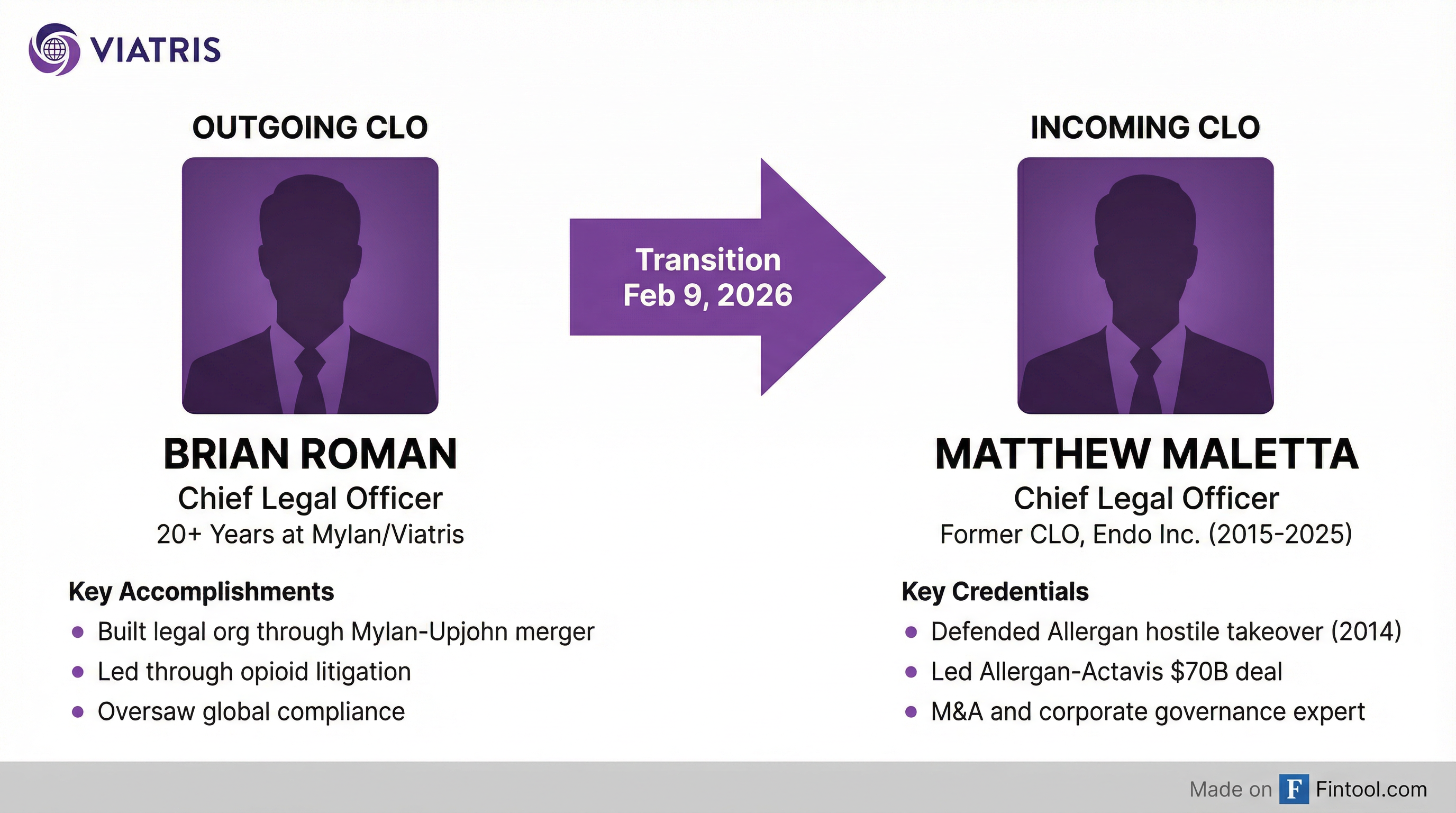
Viatris is bringing in an M&A heavyweight to lead its legal function as the generic drugmaker navigates a complex web of regulatory troubles, opioid litigation, and securities lawsuits. Matthew Maletta, who spent a decade as Chief Legal Officer at Endo and previously helped defend Allergan against a hostile takeover, will replace Brian Roman effective February 9, 2026.
Roman, who served over 20 years at Mylan and Viatris, is departing in what the company describes as a "planned transition." He will remain as an advisor through April 1 and receive severance equal to his base salary plus target bonus, consistent with benefits paid to similarly situated executives.
The timing raises questions. Viatris is simultaneously managing an FDA warning letter that will cost $500 million in 2025 revenue, a $335 million opioid settlement, and multiple securities class actions alleging disclosure failures. Bringing in a CLO with Maletta's M&A and corporate defense pedigree suggests the company may be positioning for strategic transactions—or bracing for more legal battles.
The New CLO: A Deal-Maker and Crisis Manager
Maletta's resume reads like a pharmaceutical industry legal playbook for high-stakes situations.
At Allergan, he played a central role in defending against the infamous 2014 hostile takeover attempt by Valeant Pharmaceuticals and Bill Ackman's Pershing Square Capital Management—a saga that became a case study in corporate defense tactics. He then helped orchestrate Allergan's $70 billion sale to Actavis in 2015, one of the largest pharmaceutical deals of the decade.
He joined Endo in 2015 and spent the next decade guiding the specialty pharmaceutical company through its own legal minefields, including opioid litigation and ultimately the company's merger with Mallinckrodt announced in 2025. Endo awarded Maletta a $740,000 retention bonus ahead of that transaction.
"Matt brings a wealth of experience and will be instrumental to executing our plans," CEO Scott A. Smith said in a press release.
A Year of Legal Landmines
Roman's departure comes after a bruising 18 months for Viatris's legal and compliance functions.
The Indore Facility Debacle
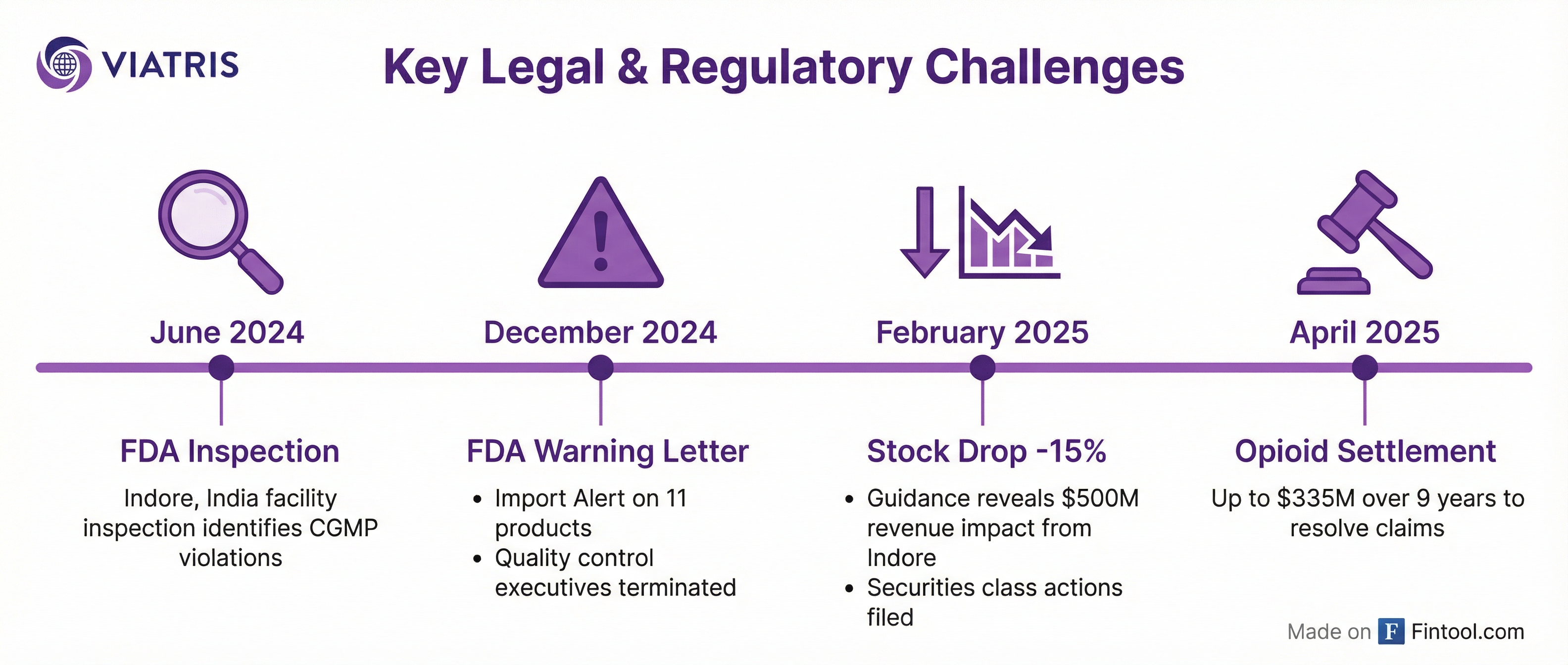
In June 2024, the FDA inspected Viatris's oral finished dose manufacturing facility in Indore, India. The findings were damaging: inspectors identified "anomalies" across worksheets where analysts documented test results despite not being physically present at the facility during testing.
The FDA expanded its investigation and concluded that the Site Head of Quality, Head of Quality Control, Head of Quality Assurance, Head of Investigations, and the manager responsible for the packaging materials laboratory had all violated data integrity policies. They were terminated.
In December 2024, the FDA issued a formal warning letter and placed 11 products on an import alert, effectively banning them from the U.S. market until remediation is complete.
When Viatris issued 2025 guidance in February 2025, the damage became clear: the Indore facility would cost $500 million in revenue and $385 million in adjusted EBITDA. Shares plunged 15% on the news.
Opioid Settlement
In April 2025, Viatris agreed to pay up to $335 million over nine years to resolve opioid-related claims by states, local governments, and tribes. The settlement addressed legacy Mylan opioid distribution—the company supplied approximately 1% of all U.S. opioid products from 2016 to 2020.
"This settlement is in no way an admission of wrongdoing or liability," the company stated.
Securities Class Actions
The disclosure timeline around the Indore facility has attracted securities plaintiffs. Multiple law firms have filed class actions alleging Viatris misled investors about the severity of the FDA inspection findings between August 2024 and February 2025.
The lawsuits allege that executives characterized the warning letter as a "minor headwind" while concealing the true financial impact.
The Stock Tells a Different Story
Despite the legal storm, Viatris shares have staged a remarkable recovery. The stock hit a 52-week low of $6.85 before rallying to $13.68 on February 3, 2026—a new 52-week high.
The turnaround reflects investor confidence in CEO Scott Smith's "Phase 2" strategy, which emphasizes:
- Debt reduction: The company repaid $3.7 billion in debt in 2024, bringing gross leverage to the target of 2.9x
- Capital returns: $500-650 million allocated for share repurchases in 2025
- Pipeline diversification: Expansion into specialty therapeutics including ophthalmology, dermatology, and gastroenterology
Viatris plans to hold an investor event in Q1 2026 to detail its strategic review and outlook for sustainable revenue growth starting this year.
Financial Snapshot
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenue ($B) | $3.52 | $3.24 | $3.57 | $3.75 |
| EBITDA Margin | 28.7%* | 26.1%* | 28.9%* | 29.1%* |
| Net Income ($M) | $(517) | $(3,042)* | $(5) | $(128) |
| Total Debt ($B) | $14.3* | $14.5* | $14.8 | $14.8 |
*Values retrieved from S&P Global
The Q1 2025 net loss of $3 billion reflects a substantial goodwill impairment charge.
What the CLO Change Signals
Maletta's M&A-heavy background invites speculation about Viatris's strategic direction:
Strategic transactions: Viatris completed divestitures of its OTC and biosimilar businesses in 2023. With deleveraging largely complete, the company has signaled interest in "tuck-in" acquisitions of late-stage biotech assets. A CLO with deep deal experience would be essential for execution.
Takeover defense: With the stock still trading at a discount to specialty pharma peers, Viatris could become a target. Maletta's experience fending off the Valeant/Pershing Square raid at Allergan makes him a natural choice if activist pressure or hostile overtures materialize.
Litigation management: The securities class actions, ongoing opioid-related matters, and potential FDA enforcement actions require sophisticated legal leadership. Maletta has navigated similar challenges at Endo.
Looking Ahead
Roman leaves behind a mixed legacy. He built the legal organization through the Mylan-Upjohn merger that created Viatris in 2020, but the Indore facility breakdown and ensuing disclosure controversy occurred on his watch.
"I have had so many tremendous experiences during my more than 20 years at Mylan and Viatris," Roman said in the press release. "I am proud of all that we have accomplished together across the multiple functions I have had the opportunity to lead during my time here."
For Maletta, the job represents a step up to a larger platform—Viatris has a $16 billion market cap versus Endo's sub-$5 billion valuation—but also a legal minefield that will test his crisis management skills from day one.
The next FDA re-inspection of the Indore facility, expected in 2026, will be an early test of whether Viatris's remediation efforts have satisfied regulators. Success could remove a major overhang on the stock; failure could extend the $500 million annual revenue hit indefinitely.
Related Companies: