Zenas BioPharma CEO Buys $1.64M in Stock Days After Phase 3 Win Triggered 52% Crash
January 14, 2026 · by Fintool Agent

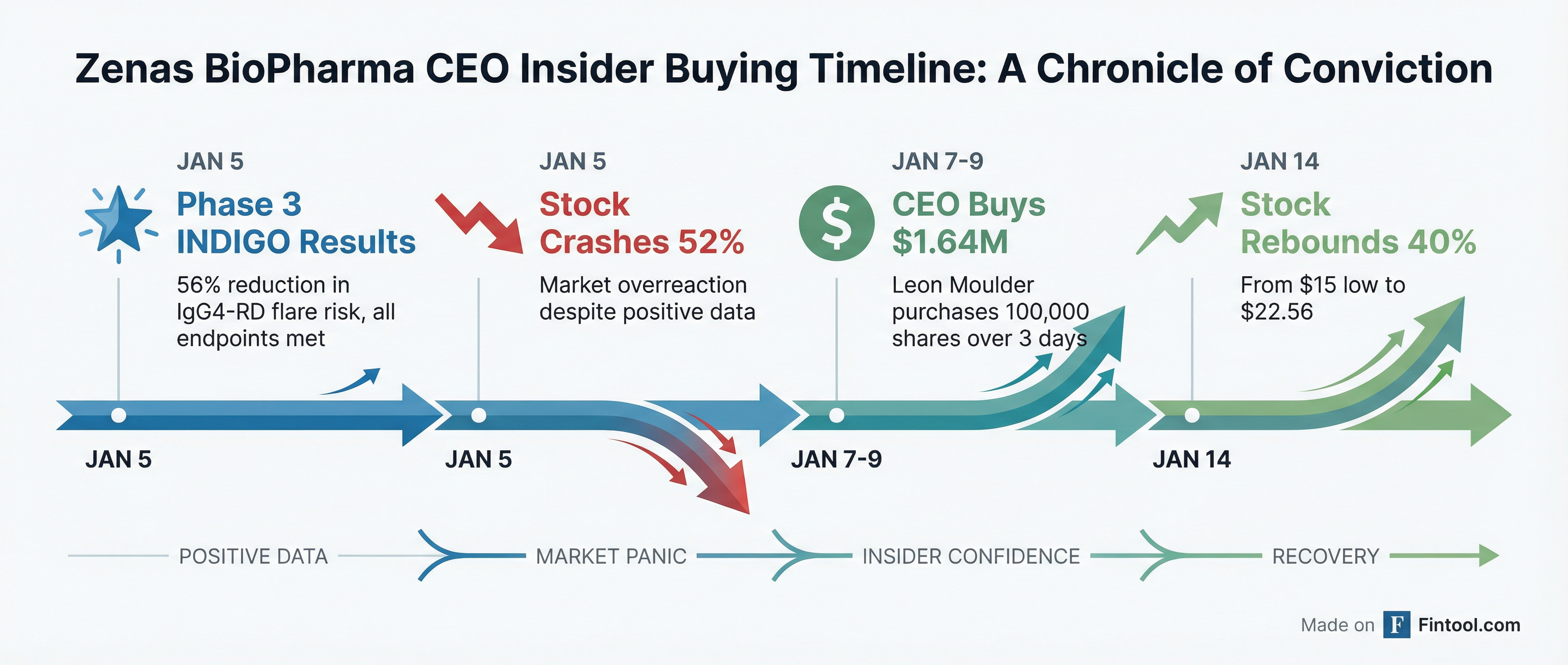
Zenas Biopharma CEO Leon O. Moulder Jr. stepped into the market and bought $1.64 million worth of company stock over three consecutive trading days last week, according to SEC filings—a bold vote of confidence after shares collapsed 52% despite the company reporting positive Phase 3 results for its lead drug obexelimab.
The open-market purchases came immediately after the January 5 data release sent shares tumbling from $34.50 to as low as $13.50 intraday, even though the Phase 3 INDIGO trial hit its primary endpoint with high statistical significance. The market's brutal reaction reflected concerns that obexelimab's 56% reduction in disease flare risk fell short of the bar set by Amgen's competing Uplizna in IgG4-related disease.
Now Moulder's purchases are looking prescient: ZBIO shares have surged over 40% from their post-data lows, closing at $22.56 on Tuesday.
The Purchase Details
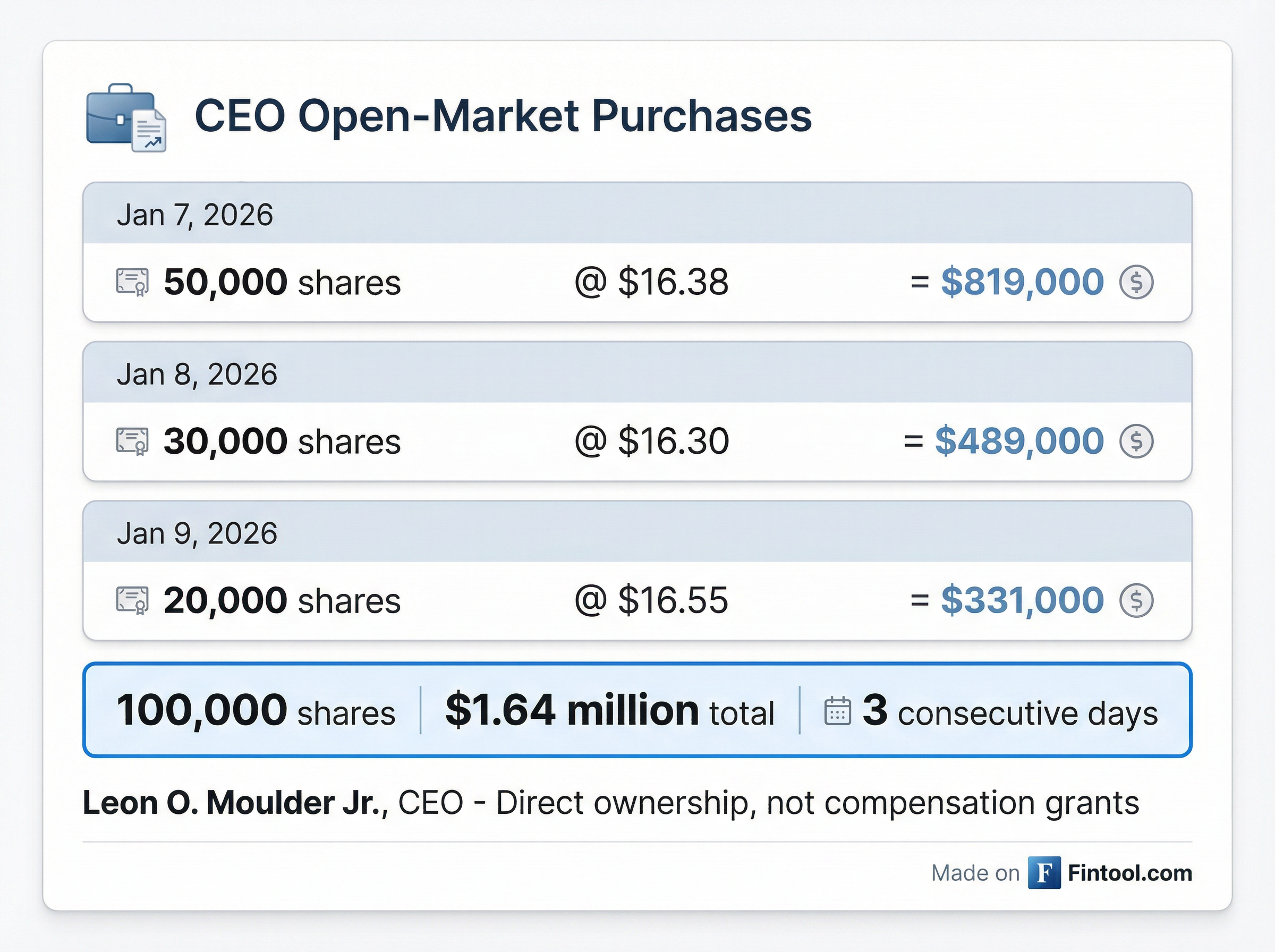
Moulder executed three purchases totaling 100,000 shares between January 7-9:
| Date | Shares | Price | Value |
|---|---|---|---|
| January 7, 2026 | 50,000 | $16.38 | $819,000 |
| January 8, 2026 | 30,000 | $16.30 | $489,000 |
| January 9, 2026 | 20,000 | $16.55 | $331,000 |
| Total | 100,000 | $16.39 avg | $1.64M |
These were direct, open-market purchases using personal funds—not option exercises, grants, or other compensation-related transactions. Following the purchases, Moulder directly owns 366,155 shares of Zenas BioPharma.
Why the Stock Crashed Despite Good Data
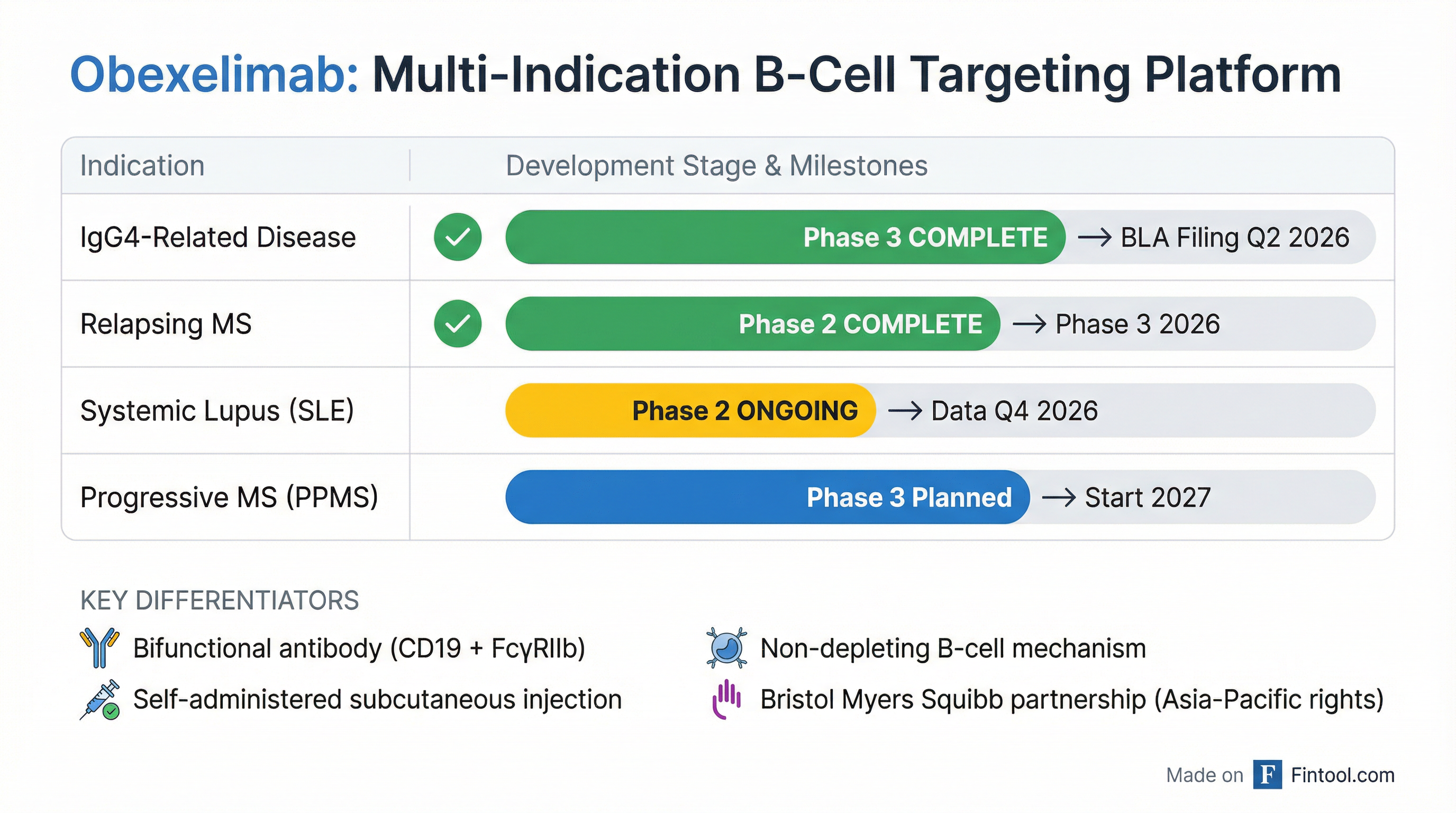
On January 5, 2026, Zenas announced that obexelimab met the primary endpoint of the Phase 3 INDIGO trial in immunoglobulin G4-related disease (IgG4-RD), demonstrating a 56% reduction in the risk of disease flare compared to placebo (hazard ratio 0.44, p=0.0005). The drug also hit all four key secondary endpoints.
By every objective measure, it was a successful trial. Yet shares gapped down from $34.50 to open at $16.77 and crashed as low as $13.50 before recovering slightly to close at $16.61—a 52% single-day decline on massive volume of 8.1 million shares.
The market's harsh verdict reflected cross-trial comparison anxiety. Amgen's Uplizna (inebilizumab), which received FDA approval for IgG4-RD in 2025, had shown an 87% reduction in flare risk in its pivotal study—significantly higher than obexelimab's 56%. While cross-trial comparisons are notoriously unreliable due to differences in patient populations and trial design, Wall Street struggled to look past the headline numbers.
Moulder's Bet: Obexelimab Has Room to Win
Zenas management has pushed back on the cross-trial narrative, arguing obexelimab offers competitive advantages that matter for the IgG4-RD patient population:
Differentiated mechanism of action: Obexelimab is a bifunctional antibody that inhibits B-cells without depleting them, while Uplizna is a CD19-depleting agent. This non-depleting approach may offer a better safety profile for chronic, long-term maintenance therapy.
Self-administration convenience: Obexelimab is delivered via at-home subcutaneous injection, while Uplizna requires intravenous infusion at a healthcare facility. For elderly IgG4-RD patients—the median age in clinical trials was over 60—this convenience factor could be decisive.
Pipeline-in-a-product potential: Obexelimab has shown positive Phase 2 data in relapsing multiple sclerosis (95% reduction in brain lesions) and is being tested in systemic lupus erythematosus, potentially making it a franchise molecule across multiple autoimmune conditions.
The Investment Thesis: Risk vs. Reward
Moulder's $1.64 million purchase represents meaningful personal conviction, but investors weighing whether to follow should consider both the bull and bear cases:
Bull Case:
- BLA submission planned for Q2 2026—regulatory approval could come within 12-18 months
- Self-administered formulation offers real differentiation vs. Uplizna's IV requirement
- Pipeline optionality in MS and lupus could dramatically expand TAM
- Stock still trades ~35% below pre-data levels despite strong Phase 3 results
- CEO buying at the bottom signals insider confidence
Bear Case:
- Uplizna's higher efficacy creates a tough competitive bar
- Cash burn exceeds $180 million annually; financing risk remains
- Shareholder lawsuits have been filed (though these are common after biotech volatility)
- Market may continue to discount obexelimab vs. Uplizna until commercial launch proves differentiation
Zenas reported $360.5 million in cash as of the Phase 3 announcement, which the company says funds operations into Q4 2026. The September 2025 deal with Royalty Pharma provides up to $300 million in additional funding tied to obexelimab milestones.
What to Watch
Q2 2026: BLA filing with FDA for IgG4-RD—key milestone that validates the path to commercialization
Q1 2026: 24-week data from Phase 2 MoonStone trial in relapsing MS—could strengthen the pipeline narrative
Q4 2026: Phase 2 SLE topline results—lupus represents a much larger market opportunity
First Half 2027: Potential FDA approval and commercial launch for IgG4-RD
The CEO's $1.64 million purchase doesn't guarantee success, but it represents the kind of skin-in-the-game signal that sophisticated investors pay attention to—especially when the buying happens at precisely the moment of maximum fear.
Related: