Earnings summaries and quarterly performance for AMGEN.
Executive leadership at AMGEN.
Robert A. Bradway
Chief Executive Officer and President
David M. Reese
Executive Vice President and Chief Technology Officer
James E. Bradner
Executive Vice President, Research and Development
Murdo Gordon
Executive Vice President, Global Commercial Operations
Peter H. Griffith
Executive Vice President and Chief Financial Officer
Board of directors at AMGEN.
Amy E. Miles
Director
Brian J. Druker
Director
Charles M. Holley, Jr.
Director
Ellen J. Kullman
Director
Greg C. Garland
Director
Mary E. Klotman
Director
Michael V. Drake
Director
Robert A. Eckert
Lead Independent Director
S. Omar Ishrak
Director
Tyler Jacks
Director
Wanda M. Austin
Director
Research analysts who have asked questions during AMGEN earnings calls.
Salveen Richter
Goldman Sachs
8 questions for AMGN
Terence Flynn
Morgan Stanley
8 questions for AMGN
Yaron Werber
TD Cowen
8 questions for AMGN
David Amsellem
Piper Sandler Companies
7 questions for AMGN
Christopher Schott
JPMorgan Chase & Co.
6 questions for AMGN
Jay Olson
Oppenheimer & Co. Inc.
6 questions for AMGN
Courtney Breen
AllianceBernstein
5 questions for AMGN
Evan Seigerman
BMO Capital Markets
5 questions for AMGN
Matthew Phipps
William Blair
5 questions for AMGN
Umer Raffat
Evercore ISI
5 questions for AMGN
David Risinger
Leerink Partners
4 questions for AMGN
Michael Yee
Jefferies
4 questions for AMGN
Mohit Bansal
Wells Fargo & Company
4 questions for AMGN
Alexandria Hammond
Wolfe Research
3 questions for AMGN
Gregory Renza
RBC Capital Markets
3 questions for AMGN
Alex Hammond
Sidoti & Company, LLC
2 questions for AMGN
Carter L. Gould
Barclays
2 questions for AMGN
Chris Schott
JPMorgan Chase & Company
2 questions for AMGN
Louise Chen
Cantor Fitzgerald
2 questions for AMGN
Christopher Raymond
Piper Sandler
1 question for AMGN
Conor MacKay
BMO Capital Markets
1 question for AMGN
Geoff Meacham
Citigroup Inc.
1 question for AMGN
James Shin
Analyst
1 question for AMGN
Luca Issi
RBC Capital Markets
1 question for AMGN
Michael DiFiore
Evercore ISI
1 question for AMGN
Michael Gee
UBS Financial Services Inc.
1 question for AMGN
Mike DiFiore
Evercore ISI
1 question for AMGN
Olivia Brayer
Cantor
1 question for AMGN
Sadia Rahman
Wells Fargo
1 question for AMGN
Timothy Anderson
BofA Securities
1 question for AMGN
Trung Huynh
UBS Group AG
1 question for AMGN
Recent press releases and 8-K filings for AMGN.
- On February 17, 2026, Amgen sold four tranches of senior unsecured notes totaling $4.0 billion, comprising $1.0 billion of 4.200% notes due 2031, $1.75 billion of 4.850% notes due 2036, $0.5 billion of 5.500% notes due 2046 and $0.75 billion of 5.650% notes due 2056, generating net proceeds of approximately $3.961495 billion.
- The notes pay interest semi-annually in arrears each February 19 and August 19, rank pari passu with all existing and future senior unsecured debt and are effectively subordinated to any secured obligations of Amgen’s subsidiaries.
- Holders have an optional put on a change-of-control event at 101% of principal plus accrued interest, and the company may optionally redeem notes at a make-whole premium prior to specified call dates.
- Amgen reaffirmed its oncology R&D framework to focus on transformative therapies in both hard-to-treat solid tumors and hematologic malignancies, anchored by its T-cell engager (BiTE) and precision small-molecule platforms.
- IMDELLTRA (DLL3 BiTE) has full U.S. approval in second-line small cell lung cancer, is administered at over 1,600 U.S. sites, and is being evaluated in three additional Phase 3 trials (first-line metastatic, maintenance and limited stage), plus subcutaneous and extended-interval dosing studies to improve access.
- BLINCYTO’s development includes a subcutaneous formulation and earlier-line B-ALL trials (e.g., Golden Gate in patients ≥55 years), with Phase II studies underway in SLE and refractory rheumatoid arthritis, aiming to enhance convenience and expand indications.
- Xaluritamig (STEAP1 BiTE) is advancing in Phase III for post-taxane and pre-taxane metastatic castration-resistant prostate cancer—designed to demonstrate overall survival benefit versus chemotherapy without requiring biomarker gating—and is also being explored in Ewing sarcoma.
- Amgen reaffirmed its R&D framework targeting differentiated, transformative therapies across hard-to-treat solid tumors and select hematologic malignancies, with two core modalities—T-cell engagers and precision small molecules—and selective ADC investments leveraging its biologics and chemistry strengths.
- The DLL3-targeting BiTE IMDELLTRA received full approval in second-line or later small cell lung cancer, now administered at over 1,600 U.S. sites (mostly community); three pivotal phase III trials in first-line metastatic, maintenance and limited-stage settings are ongoing globally.
- KRAS G12C inhibitor LUMAKRAS is approved in second-line NSCLC and third-line colorectal cancer; ongoing studies include CodeBreaK 202 (chemotherapy ± LUMAKRAS vs. chemo + pembrolizumab in first-line NSCLC) and a first-line CRC trial of LUMAKRAS + Vectibix + FOLFIRI, which showed ~55% ORR and >90% DCR in phase II.
- STEAP1-targeting BiTE xaluritamig is being advanced in prostate cancer with survival-driven pivotal trials both pre- and post-taxane (chemo-free in the former) and in relapsed/refractory Ewing sarcoma, aiming to fill unmet need without biomarker gating.
- Amgen emphasizes transformative R&D in T-cell engagers and precision small molecules across hard-to-treat solid tumors and hematological malignancies, focusing on high-impact modalities over marginal benefit.
- IMDELLTRA, approved in second-line extensive-stage small cell lung cancer and administered at over 1,600 U.S. sites, is entering phase 3 trials in first-line, maintenance, and limited-stage settings, with exploratory studies in neuroendocrine prostate cancer.
- LUMAKRAS is marketed in second-line non-small cell lung cancer and third-line colorectal cancer; pivotal phase 3 trials are underway in first-line lung (CodeBreaK 02) and first-line colorectal cancer using triplet combinations.
- Xaluritamig is being positioned for overall survival endpoints in post-taxane and pre-taxane metastatic prostate cancer as a monotherapy and chemo-free option, and is also in phase 1b development for STEAP1-positive Ewing sarcoma.
- UPLIZNA to enter Phase III trials in autoimmune hepatitis and CIDP in 2026; CIDP prevalence in the U.S. is ≈35,000 with 7,000–10,000 new cases annually; AIH population considered larger though less defined.
- Daxdilimab (ILT-7 targeting) achieved Phase II success in discoid lupus, meeting primary and key secondary endpoints, supporting advancement to Phase III in cutaneous lupus.
- Dazodalibep, a CD40 ligand inhibitor, is in two Phase III trials for Sjögren’s syndrome—one in moderate–severe systemic disease and one in high symptomatic burden—after Phase II efficacy across both patient subsets.
- TEZSPIRE approved for chronic rhinosinusitis with nasal polyps showed significant reductions in polyp severity, surgery needs, and steroid use; pipeline expands to COPD and eosinophilic esophagitis (Phase III readouts H2 2026) and an inhaled TSLP fragment (AMG 104) completing Phase II.
- UPLIZNA (anti-CD19) to enter Phase III studies in autoimmune hepatitis (AIH) and chronic inflammatory demyelinating polyneuropathy (CIDP) later in 2026; U.S. CIDP prevalence is estimated at 35,000 with 7,000–10,000 new annual cases, while AIH’s pool is larger but not precisely quantified.
- Daxdilimab (anti-ILT-7) met its primary and key secondary endpoints in a Phase II study for discoid lupus, and Amgen is evaluating Phase III design and broader cutaneous lupus indications.
- Dazodalibep, a CD40-ligand inhibitor, has completed enrollment in two Phase III trials targeting moderate-to-severe systemic and high-symptomatic/low-systemic Sjögren’s syndrome following positive Phase II results.
- TEZSPIRE (anti-TSLP) gained approval in chronic rhinosinusitis with nasal polyps after the Phase III Waypoint trial showed significant polyp reduction, near-elimination of surgery need, and reduced steroid use versus placebo—leveraging an upstream mechanism versus IL-4/5/13 inhibitors.
- Amgen anticipates Phase III readouts for TEZSPIRE in eosinophilic esophagitis (prevalence ~1 in 700 in the U.S.) and COPD in H2 2026, and is developing AMG 104, an inhaled TSLP antibody fragment, as a potential alternative to injectable biologics.
- UPLIZNA (anti-CD19) will enter Phase III trials in autoimmune hepatitis and chronic inflammatory demyelinating polyneuropathy, with an estimated U.S. CIDP population of 35,000 prevalent and 7,000–10,000 incident patients annually.
- Daxdilimab (anti-ILT-7) met its primary and key secondary endpoints in a Phase II discoid lupus study, prompting planning for a pivotal Phase III trial in cutaneous lupus manifestations.
- Dazodalibep (CD40L inhibitor) is in two Phase III trials for Sjögren’s syndrome, targeting systemic disease activity and high symptomatic burden subgroups, with readouts expected in H2 2026.
- TEZSPIRE (anti-TSLP) was approved for chronic rhinosinusitis with nasal polyps, demonstrating reduced polyp severity, surgery need and steroid use, and is being evaluated in COPD and eosinophilic esophagitis, with EoE data due H2 2026.
- The inhaled TSLP antagonist AMG 104 completed Phase I and is in Phase II for severe asthma, aiming to deliver an upstream biologic directly to the lungs, with trial completion expected H1 2026.
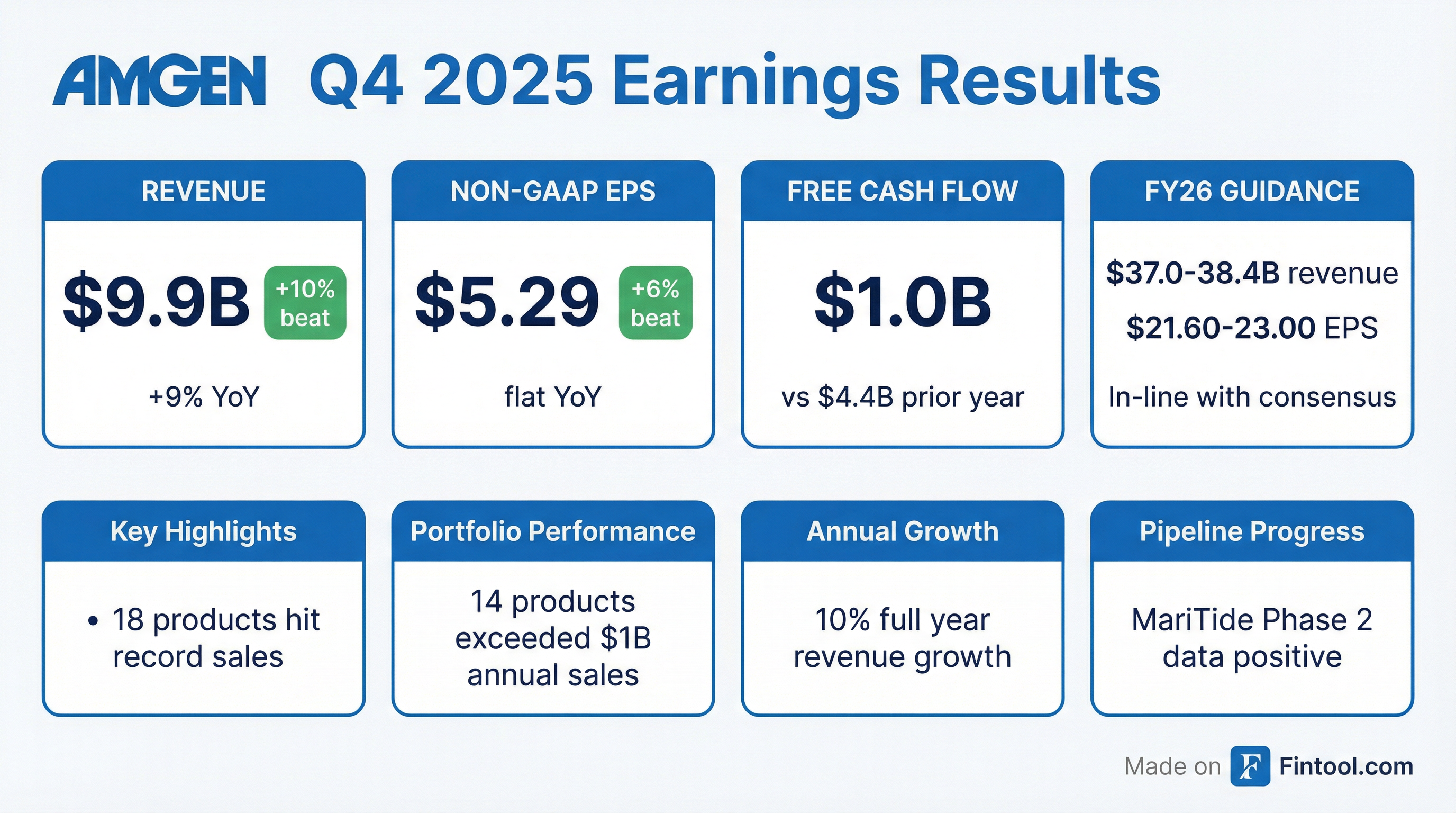
- Amgen delivered double-digit revenue and EPS growth in 2025, driven by 14 blockbuster products (>$1 B sales), 13 with double-digit growth, and 18 achieving record sales.
- Key growth drivers in 2025 included Repatha (+36% to >$3 B), Evenity (+34% to $2.1 B), Tezspire (>30%), a $5.2 B rare disease portfolio, and $3 B in biosimilars sales.
- For 2026, Amgen expects total revenues of $37.0–38.4 B and non-GAAP EPS of $21.60–23.00, noting a Q1 headwind from U.S. insurance cycles and biosimilar competition.
- R&D momentum includes six global phase III MariTide trials, the VESALIUS-CV trial showing a 25% relative risk reduction with Repatha, and full FDA approval of Imdelltra for small cell lung cancer.
- Strong full-year performance: Amgen delivered double-digit revenue and EPS growth in 2025, with 14 blockbuster products, 13 achieving double-digit sales growth, and 18 setting record results.
- Franchise drivers: Key portfolio highlights include Repatha (+36% to >$3 billion) , Evenity (+34% to $2.1 billion) , Tezspire (+52% to $1.5 billion) , rare disease (+14% to ~$5.2 billion) , innovative oncology (+11% to $8.7 billion) , and biosimilars ($3 billion).
- Financial metrics: Non-GAAP operating margin of 46%, 22% increase in R&D to $7.2 billion, $8.1 billion free cash flow, $2.2 billion capex, $6 billion of debt retired, and $2.38 quarterly dividend (+6%).
- 2026 outlook: Projected revenues of $37.0 billion–$38.4 billion and non-GAAP EPS of $21.60–$23.00, with up to $3 billion in share repurchases and $2.6 billion capex; anticipates Q1 headwinds from benefit-plan changes, biosimilar competition, and inventory adjustments.
- Q4 ’25 revenue of $9.87 B, up 9% YoY; product sales of $9.37 B, up 7%; Non-GAAP EPS of $5.29 (flat YoY).
- FY 2025 revenue of $36.75 B (+10%) and Non-GAAP EPS of $21.84 (+10%).
- 2026 guidance: revenue of $37.0 B–$38.4 B; Non-GAAP EPS of $21.60–$23.00.
- Invested $7 B in R&D (+22% YoY) while maintaining a 46% Non-GAAP operating margin; advanced pipeline with five FDA approvals and key Phase 3 milestones.
Fintool News
In-depth analysis and coverage of AMGEN.
Quarterly earnings call transcripts for AMGEN.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more