Earnings summaries and quarterly performance for Cogent Biosciences.
Executive leadership at Cogent Biosciences.
Andrew Robbins
Detailed
Chief Executive Officer and President
CEO
CP
Cole Pinnow
Detailed
Chief Commercial Officer
EK
Evan Kearns
Detailed
Chief Legal Officer and Corporate Secretary
JS
Jessica Sachs
Detailed
Chief Medical Officer
JG
John Green
Detailed
Chief Financial Officer
JR
John Robinson
Detailed
Chief Scientific Officer
Board of directors at Cogent Biosciences.
Research analysts covering Cogent Biosciences.
Recent press releases and 8-K filings for COGT.
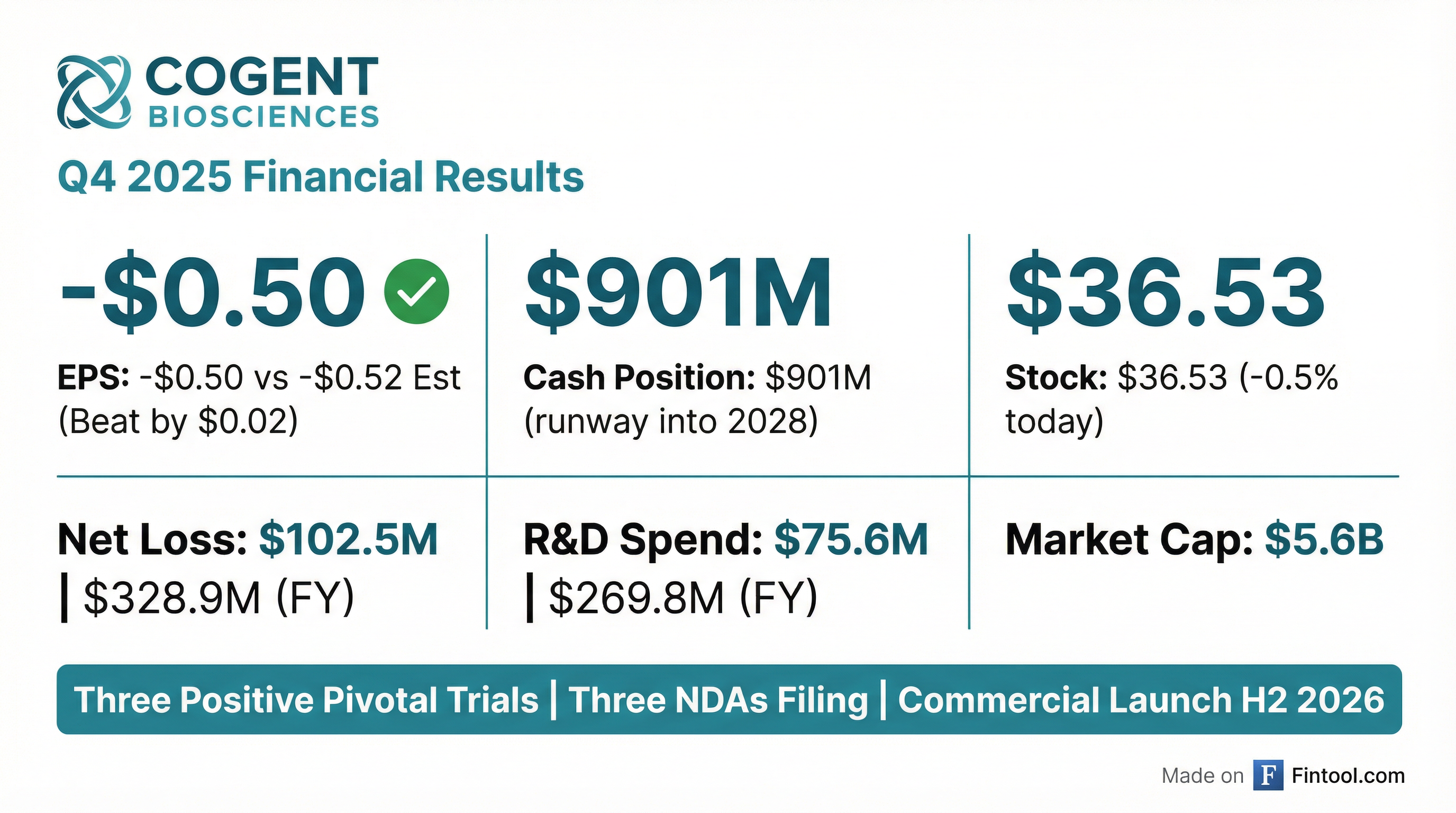
Cogent Biosciences Reports Q4 and Full Year 2025 Financial Results and Advances Bezuclastinib Regulatory Filings
COGT
Earnings
Product Launch
Guidance Update
- Cogent Biosciences has made significant regulatory progress for bezuclastinib, including the SUMMIT NDA submission for NonAdvSM in December 2025, the initiation of the PEAK NDA for 2L GIST in January 2026 (under RTOR and BTD with completion by April 2026), and the APEX NDA submission for AdvSM on track for 1H 2026.
- As of December 31, 2025, the company reported a strong financial position with $900.8 million in cash, cash equivalents, and marketable securities, which is projected to fund operations into 2028.
- For the fourth quarter and full year ended December 31, 2025, Cogent Biosciences reported a net loss of $102.5 million and $328.9 million, respectively.
- Pending FDA approval, the company anticipates launching bezuclastinib in the second half of 2026.
Feb 17, 2026, 1:12 PM
Cogent Biosciences Receives FDA Breakthrough Therapy Designation for Bezuclastinib Combination
COGT
Product Launch
New Projects/Investments
- The FDA has granted Breakthrough Therapy Designation (BTD) to Cogent Biosciences' bezuclastinib in combination with sunitinib for patients with Gastrointestinal Stromal Tumors (GIST) who have received prior treatment with imatinib.
- This BTD is based on PEAK trial results which demonstrated a 50% reduction in the risk of disease progression or death and a median progression-free survival (mPFS) of 16.5 months for the bezuclastinib combination, compared to 9.2 months for sunitinib monotherapy.
- The FDA has also agreed to accept Cogent's New Drug Application (NDA) for bezuclastinib under its Real-Time Oncology Review (RTOR) program, and the company is on track to complete the NDA submission in April 2026.
Jan 26, 2026, 1:00 PM
Cogent Biosciences Provides Update on Bezuclastinib Filings, Launch Plans, and Financial Position
COGT
Product Launch
Guidance Update
New Projects/Investments
- Cogent Biosciences' lead asset, bezuclastinib, had three positive pivotal trial readouts in 2025 for gastrointestinal stromal tumor (GIST) and systemic mastocytosis. The NDA for non-advanced systemic mastocytosis was submitted in late December, with the remaining two NDAs for GIST and advanced systemic mastocytosis expected by the first half of 2026, targeting a U.S. launch in the second half of 2026.
- The company reported a strong financial position with approximately $900 million on its balance sheet as of early 2026, providing a cash runway deep into 2028.
- Bezuclastinib targets significant market opportunities, with systemic mastocytosis representing an $8 billion annual market and second-line GIST a potential $4 billion market alone. The drug is protected by intellectual property into 2039, potentially extending to 2043.
- Cogent is also advancing its pipeline, with a pan-KRAS inhibitor and a JAK2 V617F inhibitor on track for Investigational New Drug (IND) applications in 2026.
- The company plans to build a U.S. commercial team of about 100 employees for bezuclastinib and is exploring partnerships for ex-U.S. commercialization, while potentially building out its own operations in Europe.
Jan 13, 2026, 4:15 PM
Cogent Biosciences Details 2026 Bezuclastinib Launch and Pipeline Advancement
COGT
Product Launch
New Projects/Investments
Guidance Update
- Cogent Biosciences' lead asset, Bezuclastinib, achieved positive readouts from three pivotal trials in 2025 for gastrointestinal stromal tumor (GIST) and systemic mastocytosis.
- The company plans to submit all three New Drug Applications (NDAs) for Bezuclastinib in the first half of 2026, with the SUMMIT trial NDA already submitted in late December 2025, PEAK in April 2026, and APEX following rapidly, targeting a U.S. launch in the second half of 2026.
- As of early 2026, Cogent Biosciences holds approximately $900 million on its balance sheet, providing a cash runway deep into 2028.
- Bezuclastinib is positioned to address substantial market opportunities, including an estimated $8 billion annual opportunity in systemic mastocytosis and a potential $4 billion market in second-line GIST.
- The company is also progressing its pipeline, with pan-KRAS and JAK2 V617F inhibitors on track for Investigational New Drug (IND) submissions in 2026.
Jan 13, 2026, 4:15 PM
Cogent Biosciences Prepares for Bezuclastinib U.S. Launch in H2 2026
COGT
Product Launch
New Projects/Investments
Guidance Update
- Cogent Biosciences' lead asset, bezuclastinib, successfully completed three pivotal trials in 2025 for gastrointestinal stromal tumor (GIST) and systemic mastocytosis (SM).
- The company plans to submit all three New Drug Applications (NDAs) for bezuclastinib in the first half of 2026, with the SUMMIT NDA already submitted in late December, the PEAK NDA in April, and the APEX NDA following rapidly.
- Cogent is on track to launch bezuclastinib in the U.S. in the second half of 2026, initially for non-advanced systemic mastocytosis, with potential for all three indications by year-end.
- As of early 2026, Cogent reported approximately $900 million on its balance sheet, providing a cash runway deep into 2028.
- The company is also advancing its pipeline, with pan-KRAS and JAK2 V617F inhibitors on track for Investigational New Drug (IND) submissions in 2026.
Jan 13, 2026, 4:15 PM
Cogent Biosciences Outlines 2026 Catalysts and Bezuclastinib Launch
COGT
Product Launch
New Projects/Investments
Guidance Update
- Cogent Biosciences announced three positive pivotal trial wins for Bezuclastinib (PEAK, SUMMIT, APEX) in 2025, positioning it as a best-in-class KIT inhibitor with a total global market opportunity exceeding $8 billion.
- The company submitted the NDA for SUMMIT in December 2025 and plans to submit NDAs for PEAK in April 2026 and APEX in 1H 2026, targeting a commercial launch for Bezuclastinib in 2H 2026.
- Cogent Biosciences reported approximately $900 million cash at the end of 2025, providing a cash runway well into 2028.
- The company is also advancing its pipeline with pan-KRAS and JAK2 V617F inhibitors on track for IND submissions in 2026, and anticipates intellectual property protection for Bezuclastinib through 2046.
Jan 13, 2026, 4:15 PM
Cogent Biosciences Announces 2026 Commercial and Clinical Milestones
COGT
New Projects/Investments
Product Launch
Management Change
- Cogent Biosciences announced key 2026 milestones, including planned New Drug Application (NDA) submissions for bezuclastinib in GIST (April 2026) and AdvSM (1H 2026), following the December 2025 NDA submission for NonAdvSM, with commercialization anticipated in 2H 2026.
- The company also plans to submit Investigational New Drug (IND) applications in 2026 for pan-KRAS(ON) and selective JAK2 V617F inhibitors, expanding its precision therapies portfolio.
- Cogent Biosciences reported a strong financial position, starting 2026 with approximately $900 million in cash, which is expected to fund operations and commercial launches well into 2028.
- Abb Hayden was appointed as Senior Vice President, Sales, to bolster the commercial team ahead of anticipated product launches.
Jan 12, 2026, 1:00 PM
Cogent Biosciences Submits NDA for Bezuclastinib in NonAdvanced Systemic Mastocytosis
COGT
Product Launch
New Projects/Investments
- Cogent Biosciences submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for bezuclastinib in NonAdvanced Systemic Mastocytosis (NonAdvSM) on December 30, 2025.
- This submission is based on positive clinical data from the SUMMIT pivotal trial, which achieved statistical significance across all primary and key secondary endpoints and demonstrated clear clinical benefit. Bezuclastinib was also granted Breakthrough Therapy Designation by the FDA in October 2025.
- The company plans to submit two additional NDAs for bezuclastinib in Gastrointestinal Stromal Tumors (GIST) and Advanced Systemic Mastocytosis (AdvSM) in the first half of 2026.
Dec 30, 2025, 1:00 PM
Cogent Biosciences Announces Positive Clinical Trial Results and NDA Submission Plans
COGT
New Projects/Investments
Guidance Update
- Cogent Biosciences, Inc. announced positive full results from its SUMMIT clinical trial of bezuclastinib in patients with nonadvanced systemic mastocytosis (NonAdvSM) on December 6, 2025. Bezuclastinib demonstrated clinically meaningful and statistically significant improvements across primary and key secondary endpoints, including a -24.3% mean change in Total Symptom Score (TSS) at 24 weeks compared to -15.4% for placebo (p<0.001).
- The company also announced positive top-line results from its APEX clinical trial of bezuclastinib in patients with advanced systemic mastocytosis (AdvSM) on December 8, 2025. The APEX trial showed a 57% Overall Response Rate (ORR) per mIWG criteria and an 80% ORR per pure pathological response criteria.
- Based on these results, Cogent Biosciences expects to submit a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for bezuclastinib in AdvSM during the first half of 2026.
- This marks the third positive pivotal trial result for bezuclastinib in 2025, following the SUMMIT trial in NonAdvSM and the PEAK trial in GIST patients.
Dec 8, 2025, 9:05 PM
Cogent Biosciences Reports Positive SUMMIT Trial Results for Bezuclastinib in Non-Advanced Systemic Mastocytosis
COGT
- Bezuclastinib achieved its primary endpoint in the SUMMIT trial for Non-Advanced Systemic Mastocytosis (NonAdvSM), demonstrating rapid, durable, statistically significant, and clinically meaningful symptom improvement.
- Patients treated with bezuclastinib showed a mean total symptom score (TSS) reduction of -24.32 at Week 24, compared to -15.41 for placebo.
- The drug significantly reduced objective disease markers, including serum tryptase (≥50% reduction in 95.4% of patients), bone marrow mast cell burden (≥50% reduction in 88.2%), and KIT p.D816V variant allele frequency (≥50% reduction in 97.5%).
- 55% of patients receiving bezuclastinib no longer met WHO Diagnostic Criteria, and 91% achieved a Pure Pathologic Response at 24 weeks.
- An NDA submission for a broad NonAdvSM population is expected by the end of 2025, with a global annual market opportunity exceeding $3 billion.
Dec 8, 2025, 1:00 PM
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more