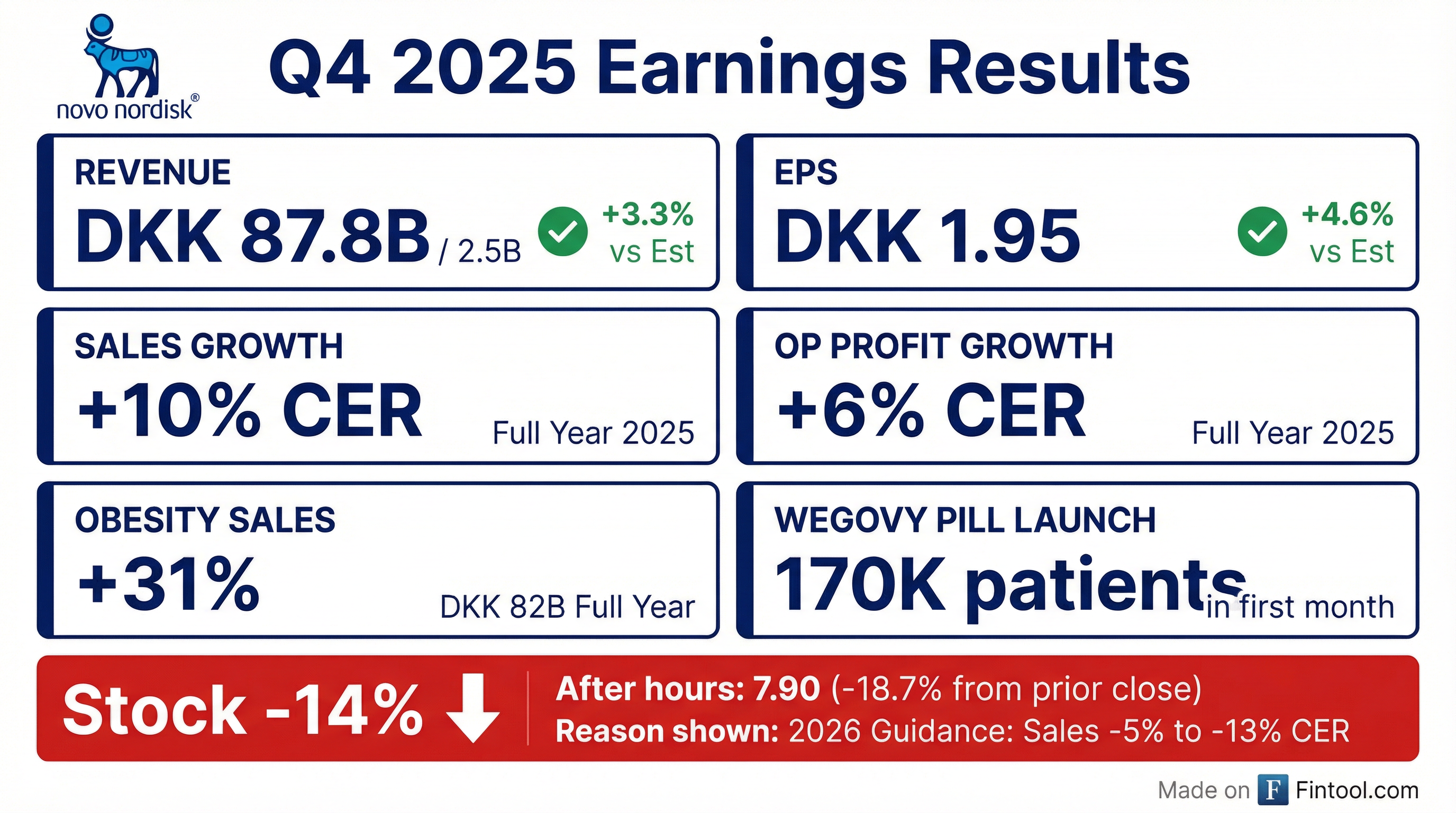
Earnings summaries and quarterly performance for NOVO NORDISK A S.
Research analysts who have asked questions during NOVO NORDISK A S earnings calls.
James Quigley
Goldman Sachs
10 questions for NVO
Sachin Jain
Bank of America
10 questions for NVO
Peter Verdult
Citigroup Inc.
9 questions for NVO
Richard Vosser
JPMorgan Chase & Co.
9 questions for NVO
Simon Baker
Redburn Atlantic
7 questions for NVO
Harry Sephton
UBS
6 questions for NVO
Michael Nedelcovych
TD Cowen
5 questions for NVO
Evan Seigerman
BMO Capital Markets
4 questions for NVO
Martin Parkhoi
SEB
4 questions for NVO
Thibault Boutherin
Morgan Stanley
4 questions for NVO
Emmanuel Papadakis
Deutsche Bank
3 questions for NVO
Jo Walton
UBS
3 questions for NVO
Rajesh Kumar
HSBC
3 questions for NVO
Callum Morris
Berenberg
2 questions for NVO
Carsten Lomberg Madsen
Danske Bank
2 questions for NVO
Carsten Lønborg Madsen
Danske Bank
2 questions for NVO
Florent Cespedes
Bernstein
2 questions for NVO
James Gordon
JPMorgan Chase & Co.
2 questions for NVO
Matt Weston
UBS
2 questions for NVO
Michael Leuchten
Jefferies
2 questions for NVO
Mike Nedelcovic
TD Cowen
2 questions for NVO
Richard Foster
JPMorgan Chase & Co.
2 questions for NVO
Yihan Li
Barclays
2 questions for NVO
Emily Field
Barclays
1 question for NVO
James Creedy
Goldman Sachs Group Inc.
1 question for NVO
Laura Hindley
Berenberg Bank
1 question for NVO
Michael Novod
Nordea
1 question for NVO
Peter Welford
Jefferies
1 question for NVO
Richard Parkes
BNP Paribas Exane
1 question for NVO
Recent press releases and 8-K filings for NVO.
- Novo Nordisk has licensed oral drug-delivery technologies from Boston startup Vivtex in a partnership potentially worth up to $2.1 billion to develop oral biologic medicines for obesity, diabetes, and related metabolic diseases.
- Vivtex will handle early research and formulation, with Novo Nordisk leading subsequent development, regulatory, manufacturing, and commercialization.
- This strategic partnership aims to rebuild Novo Nordisk's pipeline following a 63% share decline over the past 12 months and a late-stage setback for its next-generation obesity candidate.
- The deal is a move to defend market share, aligning with Novo Nordisk's recent FDA clearance for oral Wegovy and plans to cut U.S. list prices for Wegovy and Ozempic, intensifying competition in the oral obesity pill space.
- Novo Nordisk and Vivtex Corporation announced a partnership on February 25, 2026, to develop next-generation oral biologic medicines for obesity, diabetes, and associated comorbidities.
- Under the agreement, Vivtex will license select oral drug-delivery technologies to Novo Nordisk.
- Vivtex is eligible to receive up to $2.1 billion in upfront consideration, research funding, and milestone payments, along with tiered royalties on future product sales.
- The collaboration aims to enable the oral delivery of biologic drug candidates, leveraging Vivtex’s proprietary technologies to identify optimal oral formulations with improved bioavailability.
- Following research and formulation selection, Novo Nordisk will assume responsibility for global development, regulatory activities, manufacturing, and commercialization of any resulting products.
- Novo Resources Corp. is undertaking a proposed private placement to raise gross proceeds of up to approximately C$5,800,000 (approximately A$6,000,000).
- The placement involves the issue of up to 59,100,000 units and/or Chess Depository Interests (CDIs) at an issue price of C$0.10 per Share or A$0.105 per CDI.
- The issue price represents an approximate 25% discount to the market price of Shares on the Toronto Stock Exchange (TSX) (calculated as the 5-day VWAP) and an approximate 19.2% discount to the last closing price of CDIs on the ASX on February 23, 2026.
- Proceeds from the placement are expected to be used to support exploration activities at the Company's projects in the Pilbara region of Western Australia and the Belltopper Gold Project in Victoria, as well as for general working capital purposes.
- Adocia reported Q4 2025 revenue of 38 thousand euros and full-year 2025 revenue of 1.475 million euros, a decrease from 9.320 million euros in Q4 2024 and full-year 2024, which included a $10 million milestone payment.
- The company's cash position was 17.2 million euros as of December 31, 2025, an increase from 7.5 million euros at the end of 2024, providing a financial runway until early 2027.
- Stéphane Boissel was appointed Chairman of the Board of Directors, succeeding Gérard Soula, whose departure was effective February 23, 2026, and Jacky Vonderscher was co-opted as an independent director.
- Adocia announced positive Phase 3 clinical trial results for BioChaperone® Lispro in both Type 1 and Type 2 diabetes patients in China, and is advancing new platforms such as AdoXLongTM for monthly injections and AdOral® for oral peptide delivery.
- Jeito Capital was the cornerstone investor in the second closing of Alveus Therapeutics' oversubscribed Series A financing, bringing the total amount raised to $197 million as of February 24, 2026.
- The financing round included participation from Novo Holdings and other healthcare-focused funds.
- Proceeds from the financing will advance the Phase 2 clinical development of ALV-100 and support Investigational New Drug (IND) filings for early proprietary development candidates, including ALV-200, an Amylin peptide agonist.
- Alveus Therapeutics, founded in 2024, is a clinical-stage biotechnology company focused on developing next-generation therapies for obesity and metabolic diseases.
- Ksenija Pavletic, General Partner and CCO of Jeito Capital, will join the Alveus Board of Directors in conjunction with the Series A extension.
- Novo Nordisk and United Laboratories International Holdings Limited (TUL) reported positive topline results from a Chinese phase 2 trial for UBT251, a triple agonist of GLP-1, GIP, and glucagon receptors.
- The trial showed that UBT251 achieved a statistically significant mean weight loss of up to 19.7% after 24 weeks in Chinese patients with overweight or obesity, compared to 2.0% in the placebo group.
- UBT251 demonstrated a safe and well-tolerated profile, with common adverse events being mild to moderate gastrointestinal issues, consistent with incretin-based therapies.
- Novo Nordisk has initiated a global phase 1b/2a trial for UBT251, with topline data expected in 2027, and plans to start a phase 2 trial for type 2 diabetes in the second half of 2026.
- The Glucagon-like Peptide 1 (GLP-1) market is projected to grow from $22.06 billion in 2025 to $23.88 billion in 2026 at an 8.2% compound annual growth rate (CAGR), and is expected to reach $33.26 billion by 2030 with an 8.6% CAGR.
- This market expansion is primarily driven by the increasing prevalence of diabetes and obesity, rising demand for weight management therapies, and advancements in peptide drug innovation.
- Novo Nordisk A/S launched Wegovy in March 2024, which is the first once-weekly GLP-1 therapy designed for effective weight management.
- In a significant strategic move to strengthen its presence in the GLP-1 market, Novo Holdings A/S acquired Catalent Inc. for $16.5 billion in March 2024.
- The European Commission has approved a new once-weekly 7.2 mg maintenance dose of Novo Nordisk’s Wegovy for adults, following a positive scientific recommendation from the European Medicines Agency’s committee on December 12, 2025.
- This higher dose led to an average weight loss of about 21% of body weight in clinical trials, positioning it as a non-surgical alternative approaching the results of gastric sleeve surgery.
- The approval comes as Novo Nordisk faces intensifying GLP-1 competition, including cheaper compounded semaglutide offerings, and has seen its shares drop approximately 19.5% this month, leading the company to sue a former partner over plans to sell a lower-priced knockoff pill.
- The European Commission has approved a new 7.2 mg maintenance dose of Wegovy® (semaglutide) for adults with obesity, effective February 17, 2026, making it available in all 27 EU countries.
- Clinical studies demonstrated that participants on the 7.2 mg dose achieved an average 21% body weight loss.
- This higher dose provides healthcare professionals with more flexibility in tailoring treatment, and Novo Nordisk has applied for approval of a 7.2 mg single-dose pen in the EU, which could be available this year.
- Novo Nordisk A/S initiated a share repurchase programme on February 4, 2026, as part of an overall programme to repurchase up to DKK 15 billion in B shares over a 12-month period.
- A specific tranche of this programme aims to repurchase B shares for up to DKK 3.8 billion between February 4, 2026, and May 4, 2026.
- As of February 13, 2026, the company had repurchased 1,750,000 B shares for a total of DKK 533,796,640, at an average price of DKK 305.03 per B share.
- Following these transactions, Novo Nordisk holds 19,139,799 B shares as treasury shares, representing 0.4% of its share capital.
Fintool News
In-depth analysis and coverage of NOVO NORDISK A S.

Novo Nordisk Slashes Wegovy and Ozempic Prices by Up to 50%—One Day After CagriSema Trial Failure

Novo Nordisk's CagriSema Loses to Eli Lilly's Zepbound in Head-to-Head Trial, Stock Hits 5-Year Low

Novo Nordisk's CagriSema Fails to Match Eli Lilly's Zepbound in Head-to-Head REDEFINE 4 Trial

Novo Nordisk Sues Hims & Hers for Patent Infringement as FDA Cracks Down on GLP-1 Compounders

Novo Nordisk Warns of Sales Decline in 2026—Stock Plunges 12%

Novo Nordisk Cuts Wegovy Prices 50% in China as Patent Cliff Looms
Quarterly earnings call transcripts for NOVO NORDISK A S.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more