Earnings summaries and quarterly performance for NOVO NORDISK A S.
Research analysts who have asked questions during NOVO NORDISK A S earnings calls.
James Quigley
Goldman Sachs
10 questions for NVO
Sachin Jain
Bank of America
10 questions for NVO
Peter Verdult
Citigroup Inc.
9 questions for NVO
Richard Vosser
JPMorgan Chase & Co.
9 questions for NVO
Simon Baker
Redburn Atlantic
7 questions for NVO
Harry Sephton
UBS
6 questions for NVO
Michael Nedelcovych
TD Cowen
5 questions for NVO
Evan Seigerman
BMO Capital Markets
4 questions for NVO
Martin Parkhoi
SEB
4 questions for NVO
Thibault Boutherin
Morgan Stanley
4 questions for NVO
Emmanuel Papadakis
Deutsche Bank
3 questions for NVO
Jo Walton
UBS
3 questions for NVO
Rajesh Kumar
HSBC
3 questions for NVO
Callum Morris
Berenberg
2 questions for NVO
Carsten Lomberg Madsen
Danske Bank
2 questions for NVO
Carsten Lønborg Madsen
Danske Bank
2 questions for NVO
Florent Cespedes
Bernstein
2 questions for NVO
James Gordon
JPMorgan Chase & Co.
2 questions for NVO
Matt Weston
UBS
2 questions for NVO
Michael Leuchten
Jefferies
2 questions for NVO
Mike Nedelcovic
TD Cowen
2 questions for NVO
Richard Foster
JPMorgan Chase & Co.
2 questions for NVO
Yihan Li
Barclays
2 questions for NVO
Emily Field
Barclays
1 question for NVO
James Creedy
Goldman Sachs Group Inc.
1 question for NVO
Laura Hindley
Berenberg Bank
1 question for NVO
Michael Novod
Nordea
1 question for NVO
Peter Welford
Jefferies
1 question for NVO
Richard Parkes
BNP Paribas Exane
1 question for NVO
Recent press releases and 8-K filings for NVO.
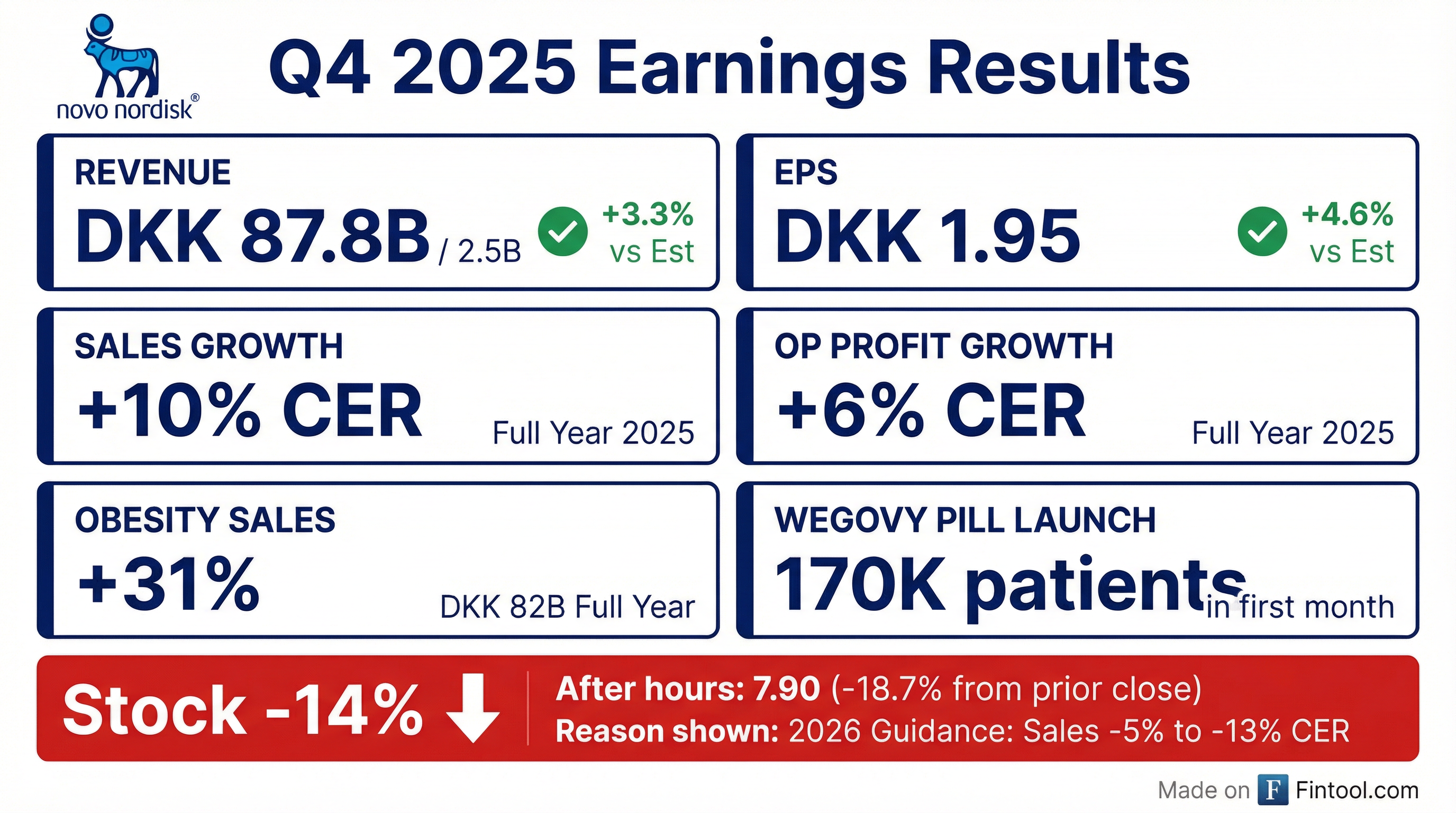
- Novo Nordisk reported a DKK 100 billion net profit and DKK 120 billion cash from operations for 2025. For 2026, the company guides for a -5% to -13% top-line decline due to pricing pressures and expects a $4.2 billion positive one-off accounting non-cash impact in Q1 2026.
- The oral Wegovy launch has been strong, achieving 16.6% weight loss and cardiovascular benefits. It recorded 50,000 new prescriptions in the U.S., reaching 170,000 patients, and its first-month launch performance was 15 times better than the Wegovy injectable and two times better than Zepbound's launch. The pill is priced at $149 and has intellectual property extending into the late 2030s.
- In 2025, Novo Nordisk allocated DKK 60 billion to CAPEX and DKK 30 billion to BD/M&A, with the bulk linked to the Akero transaction. The company will pay DKK 53 billion in dividends for 2025, marking 30 consecutive years of increased dividends, and initiated a DKK 15 billion share buyback program.
- The obesity market is highly competitive, with significant pricing pressure leading to a price-driven decline in the U.S. business. Novo Nordisk views obesity as a diversified therapy area, segmenting it by patient needs and comorbidities (e.g., MASH F4), allowing for different product offerings and pricing strategies beyond just weight loss.
- The Wegovy pill achieved 16.6% weight loss and cardiovascular benefits, with 50,000 new prescriptions and 170,000 patients in the U.S.. This launch is described as the best ever, with 15 times more first-month prescriptions than the Wegovy injectable and two times better than Zepbound's first month.
- The Wegovy pill is priced at $149 , a strategy aimed at driving volume, as price is a significant factor in market penetration. Novo Nordisk anticipates mid-teens price declines in the overall obesity market, and -10% to -15% for GLP-1s in diabetes (Ozempic).
- 2026 is expected to be a significant year with numerous readouts and initiations for diabetes and obesity treatments, including Cagrilintide, Semaglutide, CagriSema, Amycretin, and two tri-agonists. Key readouts in H2 2026 include Ziltivekimab for anti-IL-6 and regulatory approvals for Mim8 (hemophilia A) and Phase III readout for Etavopivat (sickle cell disease).
- The company is guiding for flat margins despite strong top-line growth, attributed to restructuring costs in 2025 and disciplined resourcing, including budget cuts in some areas while increasing R&D investments for top priorities.
- Novo Nordisk's Wegovy pill launch demonstrated strong initial uptake, with 50,000 new prescriptions and 170,000 patients in the U.S. during its first month, primarily attracting new patients (88% on the lowest dose). The pill offers 16.6% weight loss and cardiovascular benefits, with intellectual property extending into the late 2030s.
- The company faces significant pricing pressure in the GLP-1 market, anticipating a price-driven decline in the teens for its U.S. business, particularly for the Wegovy injectable. This competitive environment, especially from Lilly, has resulted in a preference share where competitors capture a larger portion of new patients in the U.S..
- Despite top-line declines being price-driven, Novo Nordisk is maintaining financial discipline through budget cuts across many departments while increasing investment in R&D to mitigate the impact on operating profit. The company also plans to revitalize its Ozempic brand and expand its weekly insulin in more markets.
- Dewpoint Therapeutics announced that the first patient has been dosed in a Phase 1a/2a clinical trial evaluating its lead beta-catenin program, DPTX3186, in patients with advanced solid tumors, with a focus on gastric cancer.
- DPTX3186 is a first-in-class small molecule that has received both Orphan Drug Designation and Fast Track Designation from the U.S. Food and Drug Administration (FDA).
- This clinical entry marks the first clinical validation of Dewpoint's condensate-modulating platform, which has strategic collaborations including Bayer and Novo Nordisk.
- Maziar Mike Doustdar was appointed CEO on August 7, 2025, succeeding Lars Fruergaard Jørgensen.
- Total executive remuneration for 2025 decreased by 38% to DKK 193.3 million from DKK 311.1 million in 2024, mainly due to lower payouts from short-term and long-term incentive programs.
- This reduction in incentive payouts was a result of global sales growth of 10% and operating profit growth of 6% at CER in 2025, which were significantly lower than the 26% growth for both in 2024.
- Maziar Mike Doustdar's total remuneration as CEO in 2025 was DKK 20.7 million, while former CEO Lars Fruergaard Jørgensen received DKK 22.2 million for his service period, plus DKK 42.9 million in severance and DKK 22.0 million for non-competition compensation.
- Novo Nordisk pre-released results and warned that 2026 sales and operating profit could decline 5%–13%, causing shares to plunge 15%–20% and wiping $47–50 billion off its market value.
- The company attributed the weak outlook to sharply lower U.S. pricing, intensifying competition from Eli Lilly's Zepbound, and looming patent expirations.
- Despite the negative 2026 guidance, Novo reported strong 2025 results, with full-year sales rising about 10% and operating profit up roughly 6%.
- David (Dave) Moore, Novo's U.S. head, is stepping down for personal reasons and will be replaced by Jamie (Jamey) Miller.
- Novo Nordisk reported 10% sales growth and 6% operating profit growth at constant exchange rates for 2025, with obesity care sales increasing 31% to DKK 31 billion. The company generated DKK 102 billion in net profit and DKK 120 billion in cash from operating activities.
- For 2026, the company projects adjusted sales and operating profit growth to be between -5% and -13% at constant exchange rates, primarily due to anticipated pricing headwinds, lower realized prices, and reduced Medicaid coverage for anti-obesity medications.
- The Wegovy pill, the first oral GLP-1 for obesity, received FDA approval in late 2025 and launched in the U.S. on January 5, showing encouraging early uptake with approximately 50,000 total prescriptions for the week ending January 23rd.
- Novo Nordisk plans to return over DKK 60 billion to shareholders in 2026, including a proposed total 2025 dividend of DKK 11.70 and a new share repurchase program of up to DKK 15 billion.
- Novo Nordisk delivered 10% sales growth and 6% operating profit growth in 2025. Obesity care sales increased 31% to DKK 82 billion in 2025, with Wegovy sales reaching DKK 28 billion, growing 134%.
- The company received FDA approval for the Wegovy pill by late 2025 and launched it in the U.S. on January 5, seeing encouraging early uptake with approximately 50,000 total prescriptions for the week ending January 23rd. Novo Nordisk expressed confidence in its ability to supply the U.S. market with the Wegovy pill throughout 2026.
- For 2026, Novo Nordisk anticipates adjusted sales growth and adjusted operating profit growth to be between -5% and -13% at constant exchange rates, primarily due to pricing headwinds, lower realized prices, and loss of exclusivity for semaglutide in certain international markets.
- The company announced a new share repurchase program of up to DKK 15 billion to be executed over the next twelve months. Additionally, Dave Moore, Executive Vice President of U.S. Operations, and Ludovic Helfgott, Head of Product and Portfolio Strategy, have decided to leave Novo Nordisk.
- Novo Nordisk reported 10% sales growth and 6% operating profit growth at constant exchange rates for 2025, driven by a 31% increase in obesity care sales.
- The company received FDA approval for the Wegovy pill (oral GLP-1 for obesity) in late 2025 and launched it in the US on January 5, 2026, with 50,000 total prescriptions in its first few weeks.
- For 2026, Novo Nordisk anticipates significant pricing headwinds, leading to an expected adjusted sales growth and adjusted operating profit growth of -5% to -13% at constant exchange rates.
- The board proposed a final dividend of DKK 7.95, bringing the total 2025 dividend to DKK 11.70, and approved a new share repurchase program of up to DKK 15 billion.
- Dave Moore (EVP, U.S. Operations) and Ludovic Helfgott (Head of Product and Portfolio Strategy) have decided to leave the company.
- Novo Nordisk reported sales of DKK 309.1 billion in 2025, an increase of 6% in Danish kroner and 10% at constant exchange rates (CER), while operating profit decreased by 1% in DKK but increased by 6% at CER to DKK 127.7 billion.
- Sales growth was significantly driven by Obesity care, which grew 26% in DKK (31% at CER) to DKK 82 billion in 2025, and the US FDA approved the oral Wegovy® pill on December 22, 2025, which launched on January 5, 2026.
- For 2026, the company expects adjusted sales growth of -5% to -13% at CER and adjusted operating profit growth of -5% to -13% at CER, influenced by lower realized prices and competition, but positively impacted by a USD 4.2 billion reversal of sales rebate provisions related to the 340B Drug Pricing Program.
- The Board of Directors will propose a final dividend of DKK 7.95 per share for 2025, bringing the total dividend for 2025 to DKK 11.70 per share, and has initiated a new share repurchase program of up to DKK 15 billion.
Fintool News
In-depth analysis and coverage of NOVO NORDISK A S.
Quarterly earnings call transcripts for NOVO NORDISK A S.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more