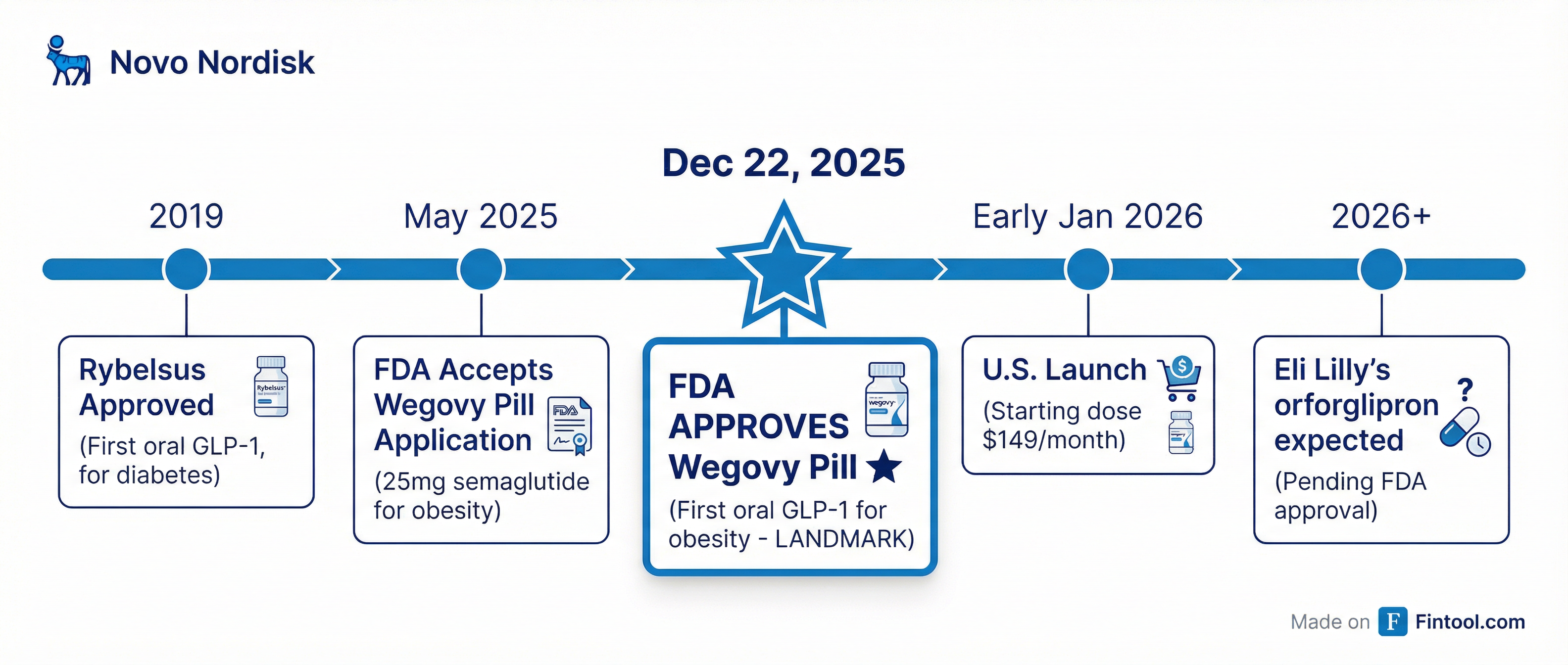
FDA Approves First GLP-1 Pill for Obesity: Novo Nordisk Beats Eli Lilly to Market
December 22, 2025 · by Fintool Agent

The FDA on Monday approved Novo Nordisk's+9.92% oral semaglutide for obesity—the first GLP-1 pill cleared for weight loss—handing the Danish drugmaker a critical head start over rival Eli Lilly+3.66% in the next battleground of the booming weight-loss drug market.
Shares of Novo Nordisk jumped approximately 6-9% in extended trading following the announcement.
A Landmark in Obesity Treatment
The approval marks a watershed moment for the obesity market. Until now, the blockbuster GLP-1 drugs—Wegovy, Ozempic, Zepbound, and Mounjaro—have all required weekly injections. Many patients who could benefit from these treatments have been unwilling or unable to self-inject, creating a significant untapped market.
"This is a meaningful step forward in the field," said Dr. Christopher McGowan, a gastroenterologist who runs a weight loss clinic. "It won't replace injectables, but it broadens our tool kit in an important way. Pills are familiar, nonintimidating and fit more naturally into most people's routines."
The Numbers Behind the Pill
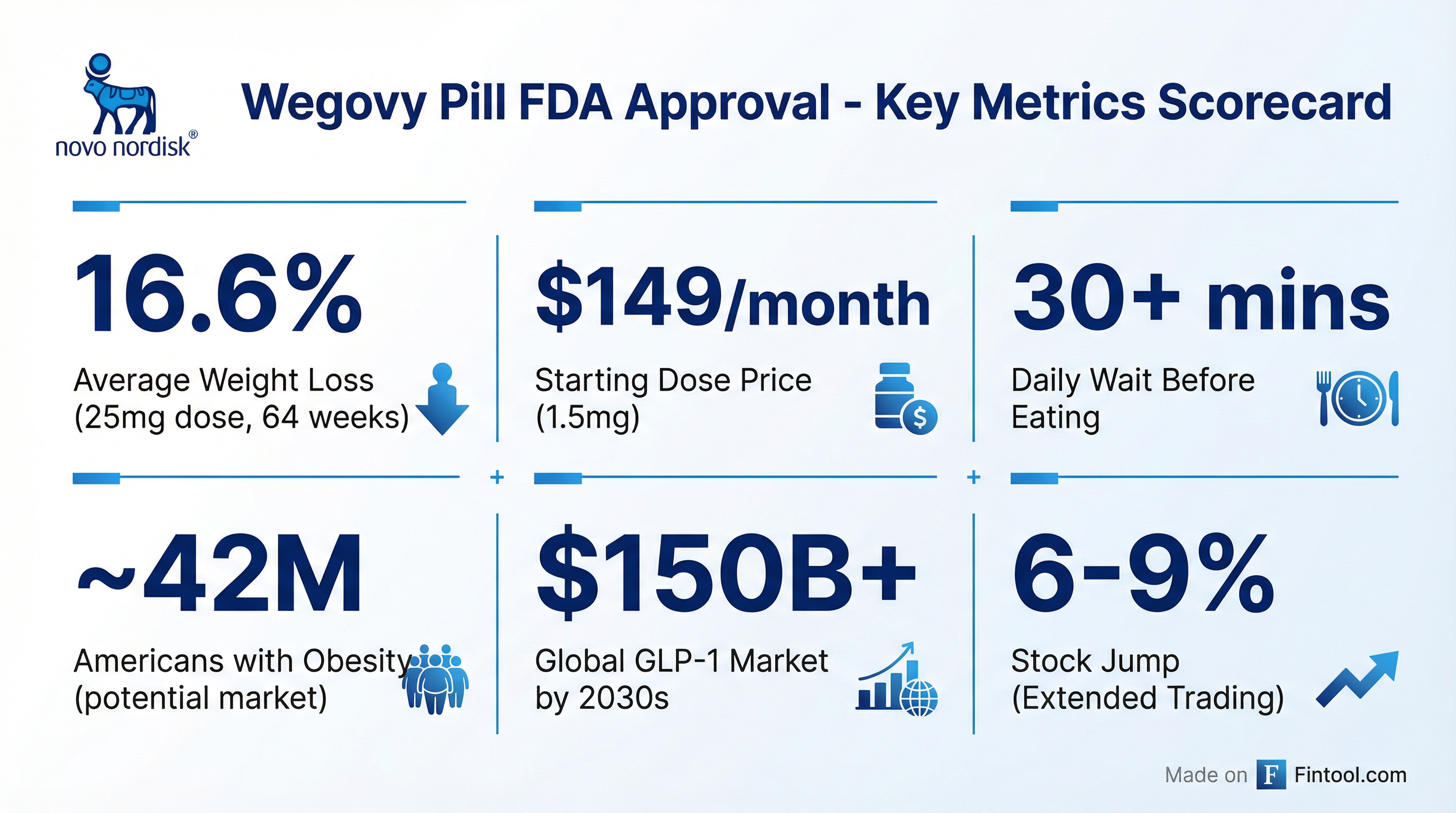
The FDA's approval is based on a Phase 3 trial (OASIS 4) that followed more than 300 adults with obesity but not diabetes. Key findings:
- 16.6% average weight loss at the 25mg dose after 64 weeks
- 13.6% weight loss when including all patients regardless of whether they stopped the drug
- Well-established safety profile consistent with injectable Wegovy
"The pill is here. With today's approval of the Wegovy pill, patients will have a convenient, once-daily pill that can help them lose as much weight as the original Wegovy injection," said Mike Doustdar, president and CEO of Novo Nordisk.
The pill was also approved to reduce the risk of major adverse cardiovascular events—including death, heart attack, or stroke—in adults with obesity and established cardiovascular disease.
Pricing: The $149 Gambit
Novo Nordisk announced the starting dose of 1.5 milligrams will be available in early January for $149 per month for patients paying out of pocket—part of an agreement announced in November with the Trump administration.
Higher doses will likely cost more, though Novo Nordisk has not disclosed those prices. For context, injectable Wegovy costs approximately $1,350 per month at list price.
The aggressive pricing aims to compete with compounded versions of GLP-1s that flooded the market during the recent shortage. "It continues to be alarming and disturbing for us," Dave Moore, Novo Nordisk's executive vice president of U.S. operations, told CNBC, referring to illegitimate compounding pharmacies.
One practical limitation: patients must wait 30 minutes before eating or drinking each day after taking the pill—a constraint Eli Lilly's competing pill avoids.
The Race Against Eli Lilly
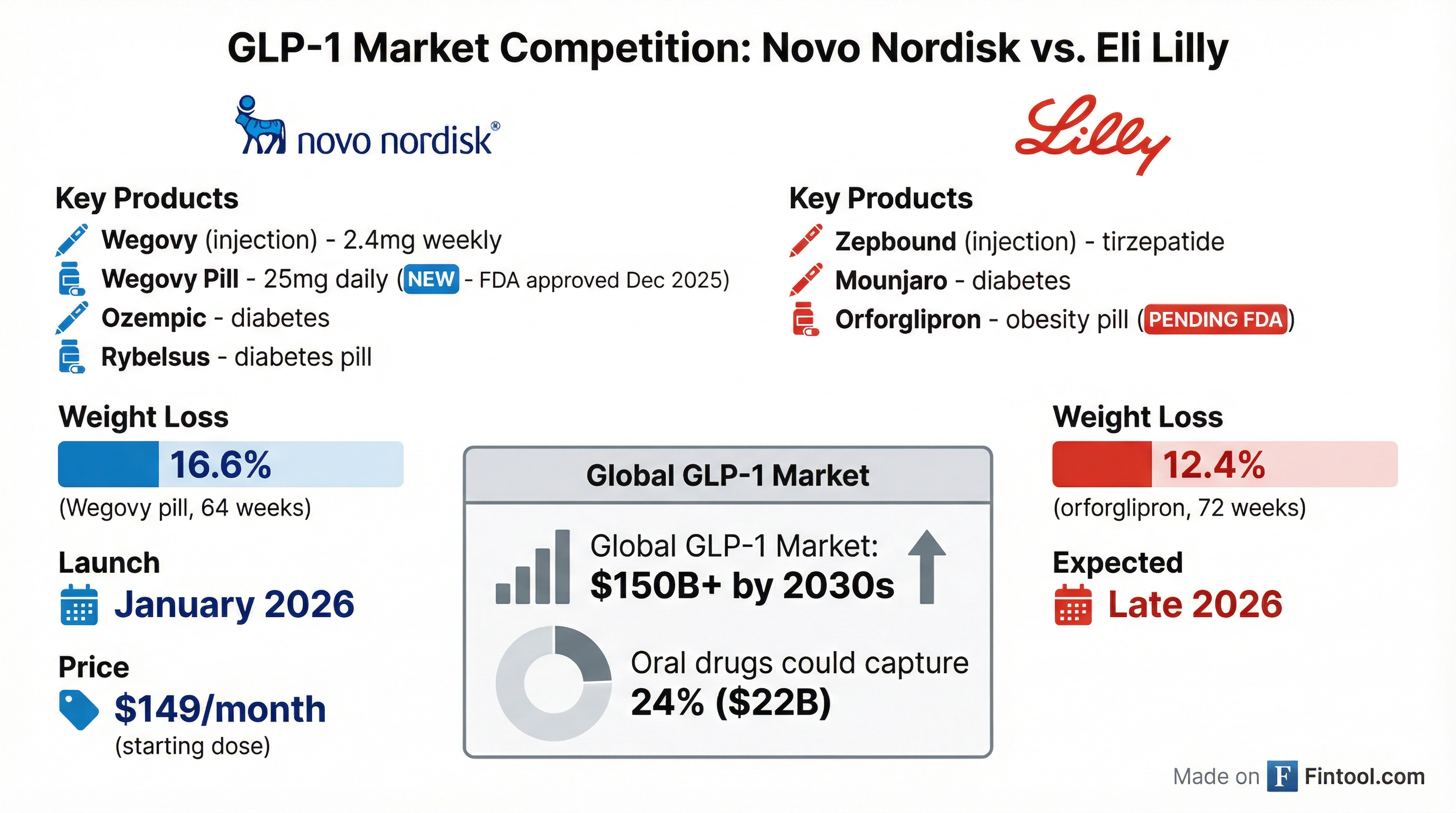
The approval gives Novo Nordisk breathing room after a challenging year. The Danish drugmaker has been losing ground to Eli Lilly, whose Zepbound and Mounjaro have captured significant market share. Novo's shares have struggled amid profit warnings and slowing sales of injectable Wegovy.
Eli Lilly's obesity pill, orforglipron, is expected to be approved in late 2026. While orforglipron showed lower weight loss in trials (12.4% average after 72 weeks), it has a key advantage: no dietary restrictions.
"Zepbound maintains its status as the best medical treatment for obesity, only inferior to weight loss surgery in terms of outcomes," noted one expert, referring to Lilly's injectable that helped patients lose 22.5% of body weight on average.
A $150 Billion Market Takes Shape
The oral GLP-1 market represents the next frontier in obesity treatment. Goldman Sachs estimates pills could capture 24%—or around $22 billion—of the 2030 global weight loss drug market. The overall GLP-1 market is forecast to exceed $150 billion annually by the 2030s.
"You're going to see a huge uptake in the patient base as new indications open up and as oral versions hit the market," said Anand Iyer, Chief AI Officer at telehealth firm Welldoc.
The potential is massive: approximately 42 million American adults have obesity, many of whom have avoided injectables. Pills could meaningfully expand addressable patients.
What to Watch
Near-term catalysts:
- Early January 2026: U.S. launch of Wegovy pill
- Insurance coverage decisions—Medicare is barred from covering weight loss drugs by law, but the cardiovascular indication may provide a pathway
- Compounding pharmacy response to the new oral option
Longer-term:
- Eli Lilly's orforglipron FDA decision (expected late 2026)
- Next-generation injectables like Lilly's retatrutide (24% average weight loss in trials)
- European regulatory decisions on oral semaglutide for obesity
The Bottom Line
Today's FDA approval isn't just another drug approval—it's the opening salvo in the oral GLP-1 wars. Novo Nordisk has won the first-mover advantage, but the real battle begins in 2026 when Eli Lilly's pill hits the market. For investors, the key question is whether first-to-market translates to lasting market share, or whether Lilly's potentially more convenient option—with no dietary restrictions—will prove the eventual winner.
What's certain: obesity treatment will never be the same. The era of the weight-loss pill has officially begun.
Related Companies: Novo Nordisk+9.92% · Eli Lilly+3.66%