Roivant Sciences (ROIV)·Q3 2026 Earnings Summary
Roivant Surges 9% After Breakthrough Phase 2 Data in Cutaneous Sarcoidosis
February 6, 2026 · by Fintool AI Agent
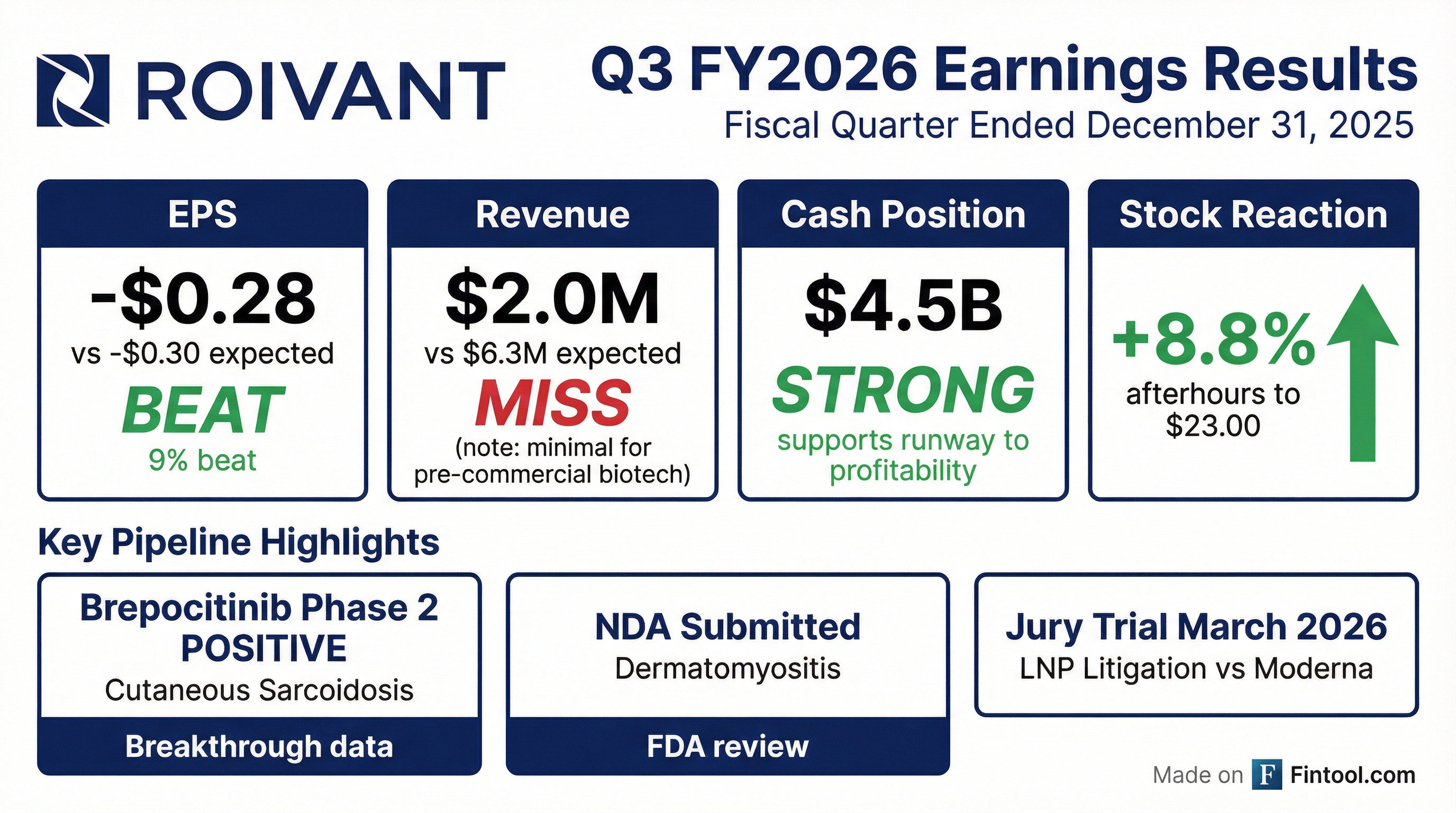
Roivant Sciences delivered a landmark quarter with breakthrough Phase 2 data for brepocitinib in cutaneous sarcoidosis—the first positive placebo-controlled trial ever for any therapy in this indication. Shares surged 8.8% afterhours to $23.00 on the news, despite a revenue miss that's typical for pre-commercial biotech. The company ended the quarter with $4.5 billion in cash, supporting runway to profitability.
Did Roivant Beat Earnings?
Roivant beat EPS expectations while missing on revenue—a common pattern for development-stage biotech where pipeline catalysts drive value:
*Values retrieved from S&P Global
The revenue miss is immaterial for Roivant's investment case—the company generates minimal revenue as a pre-commercial biotech. What matters is pipeline progress, and this quarter delivered in spades.
What Was the Breakthrough Brepocitinib Data?
The BEACON study in cutaneous sarcoidosis delivered exceptional results that CEO Matt Gline called "a breakthrough for the field."
Key Efficacy Results:
Safety: No Serious Adverse Events (SAEs) were reported. All adverse events were mild or moderate. Brepocitinib has been evaluated in more than 1,500 patients with a safety profile consistent with approved JAK1 and TYK2 inhibitors.
"The BEACON study is a watershed moment for the sarcoidosis field, and most importantly, for our patients. This is the sort of data you dream of seeing when you look at trial results—and I would call this a transformational moment for sarcoidosis." — Dr. Misha Rosenbach, MD, Professor of Dermatology and Rheumatology, Hospital of the University of Pennsylvania
Priovant plans to progress cutaneous sarcoidosis to a pivotal Phase 3 program in calendar year 2026, representing the third indication with a pivotal program for brepocitinib.
What Did Management Announce Beyond the Phase 2 Data?
This was a catalyst-rich quarter with multiple pipeline advances:
Brepocitinib (Priovant):
- NDA submitted to FDA for dermatomyositis
- Phase 3 data in non-infectious uveitis (NIU) expected H2 2026
IMVT-1402 (Immunovant):
- Potentially registrational trial in difficult-to-treat rheumatoid arthritis (D2T RA) fully enrolled, topline data H2 2026
- Proof-of-concept trial in cutaneous lupus erythematosus (CLE) data expected H2 2026
- $550 million financing completed, extending runway to launch in Graves' disease
Mosliciguat (Pulmovant):
- Phase 2 trial in pulmonary hypertension associated with ILD (PH-ILD) fully enrolled, topline data H2 2026
LNP Litigation (Genevant):
- Favorable summary judgment in U.S. Moderna case affirms section 1498 liability view
- Jury trial starts March 9, 2026
- First major international hearings expected H1 2026
How Strong Is Roivant's Balance Sheet?
Roivant's cash position remains a key strength, providing runway to profitability without additional financing needs:
The company burned approximately $133M in cash per quarter on average over the last 9 months, with R&D expenses of $165.4M and G&A of $175.1M in Q3. At current burn rates, Roivant has approximately 8+ years of runway—though expenses will likely increase as programs advance to Phase 3.
How Did R&D and G&A Expenses Change?
R&D increases were driven by:
- Mosliciguat program: +$5.7M
- Brepocitinib program: +$5.3M
- Share-based compensation: +$8.4M (Priovant Exchange Offer)
G&A increases included a $17.1M impairment loss related to HQ relocation and $17.5M in additional share-based compensation.
How Did the Stock React?
ROIV closed at $21.14 during regular trading hours (down 2.4%) but surged 8.8% afterhours to $23.00 on the Phase 2 cutaneous sarcoidosis data and NDA filing announcement.
The stock is now trading near its 52-week high of $23.91, reflecting investor optimism around the upcoming catalyst-rich 2026.
What Did Analysts Ask About?
The Q&A session revealed key strategic details not in the prepared remarks:
On Brepocitinib Pricing:
"We obviously have not decided on a price yet... those bookends [IVIG at ~$180K to Vyvgart at ~$870K] are a reasonable place to think about in terms of the pricing envelope for these indications... this will be an orphan-priced drug." — Matt Gline, CEO
On Market Opportunity: Management described cutaneous sarcoidosis as "probably a modestly smaller indication than dermatomyositis" (DM is 40K patients in treatment, 70K+ total), but emphasized high unmet need and enthusiasm for launch potential.
On D2T-RA Over-Enrollment: The IMVT-1402 trial enrolled 170 patients vs. the original target of 120 "in part just due to speed of enrollment and the level of enthusiasm from the patient and doc community."
On Competitive Positioning in Graves':
"We will have a significant lead in Graves' disease. How significant that lead is may depend a little bit on what the competitive competition does... we feel like we showed pretty conclusively in our phase 2 data that the deeper IgG suppression that we expect to deliver will matter in this population." — Matt Gline, CEO
On Phase 3 CS Design: Ben Zimmer indicated the Phase 3 would likely have "similar size per arm to the DM trial" with final design pending FDA discussions. They're "even more excited about 45 milligrams coming out of the study" after the Phase 2 data.
On Mechanism Differentiation:
"TYK2 JAK1 inhibition really does align to that through IL-12 and interferon gamma for TH1, IL-6, and IL-23 for TH17... this data really enforces that of the alignment of TYK2 JAK1 inhibition to T-cell polarization." — Ben Zimmer, CEO of Priovant
What Are the Key Risks and Concerns?
- Clinical execution risk: Multiple Phase 3 readouts in H2 2026 create binary event risk
- LNP litigation uncertainty: Moderna jury trial starts March 9, 2026—outcome is unpredictable
- Immunovant dependency: ROIV owns ~57% of IMVT; IMVT-1402 success is critical
- Cash burn trajectory: Expenses rising as multiple programs advance
- Batoclimab TED uncertainty: Both TED studies reading out H1 2026; management noted limited read-through to Graves' due to different patient populations
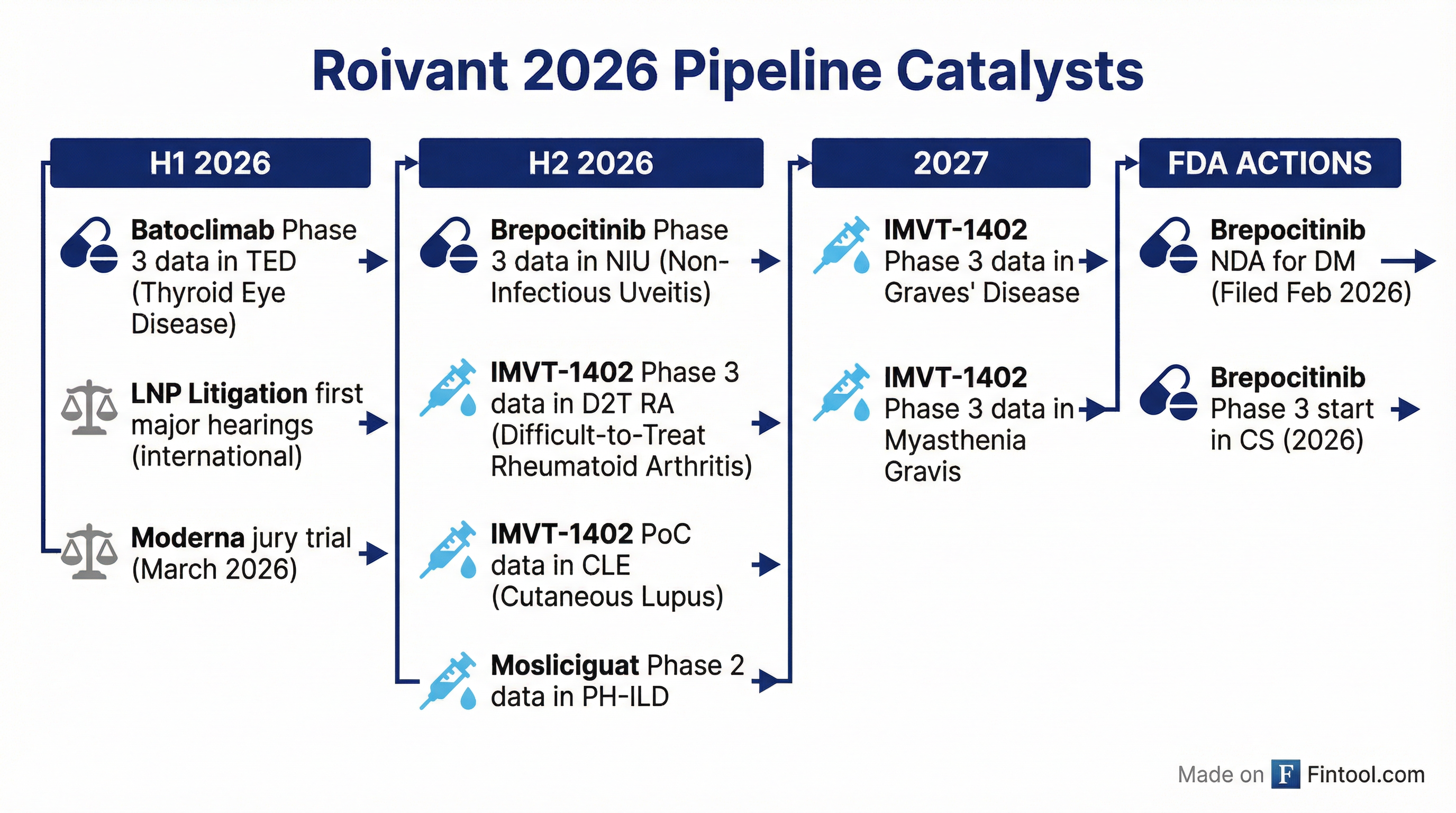
What Are the Forward Catalysts?
H1 2026:
- Batoclimab Phase 3 data in thyroid eye disease (TED)
- LNP litigation first major international hearings
- Moderna jury trial (March 2026)
H2 2026:
- Brepocitinib Phase 3 data in NIU
- IMVT-1402 Phase 3 data in D2T RA
- IMVT-1402 PoC data in CLE
- Mosliciguat Phase 2 data in PH-ILD
2027:
- IMVT-1402 Phase 3 data in Graves' disease
- IMVT-1402 Phase 3 data in myasthenia gravis
Bottom Line
Roivant delivered a transformational quarter headlined by breakthrough Phase 2 data in cutaneous sarcoidosis—the first positive placebo-controlled trial ever in this indication. With an NDA filed for dermatomyositis, multiple Phase 3 trials enrolling, and $4.5B in cash, Roivant enters 2026 with significant catalyst potential. Management's Q&A commentary revealed confidence in orphan pricing ($180K-$870K range), a "significant lead" in Graves' vs. competitors, and strong physician enthusiasm driving D2T-RA over-enrollment to 170 patients. The 9% afterhours rally reflects investor enthusiasm, but execution risk remains high with multiple binary events ahead—starting with the Moderna jury trial on March 9, 2026.