Altimmune Secures FDA Breakthrough Therapy Designation for MASH Drug Pemvidutide
January 5, 2026 · by Fintool Agent

Altimmune announced today that the FDA has granted Breakthrough Therapy Designation (BTD) to pemvidutide for the treatment of metabolic dysfunction-associated steatohepatitis (MASH), validating the drug's potential to address a massive unmet medical need and setting the stage for an accelerated path to market.
Shares of ALT surged 15% in after-hours trading, jumping from a close of $3.61 to $4.14, as investors bet that the regulatory tailwind could position the small-cap biotech as a serious challenger in the rapidly evolving MASH market—currently dominated by Madrigal Pharmaceuticals' Rezdiffra, the only FDA-approved therapy for the disease.
What Breakthrough Therapy Designation Means
The FDA grants BTD to drugs that demonstrate substantial improvement over existing therapies for serious conditions. For Altimmune, this designation brings several critical advantages:
- Intensive FDA guidance on drug development and clinical trial design
- Organizational commitment from senior FDA managers
- Rolling review allowing submission of completed sections before the full application is ready
- Potential for accelerated approval based on surrogate endpoints
The designation was granted based on 24-week data from the Phase 2b IMPACT trial, with the FDA recognizing pemvidutide's potential to provide meaningful improvement over available therapies.
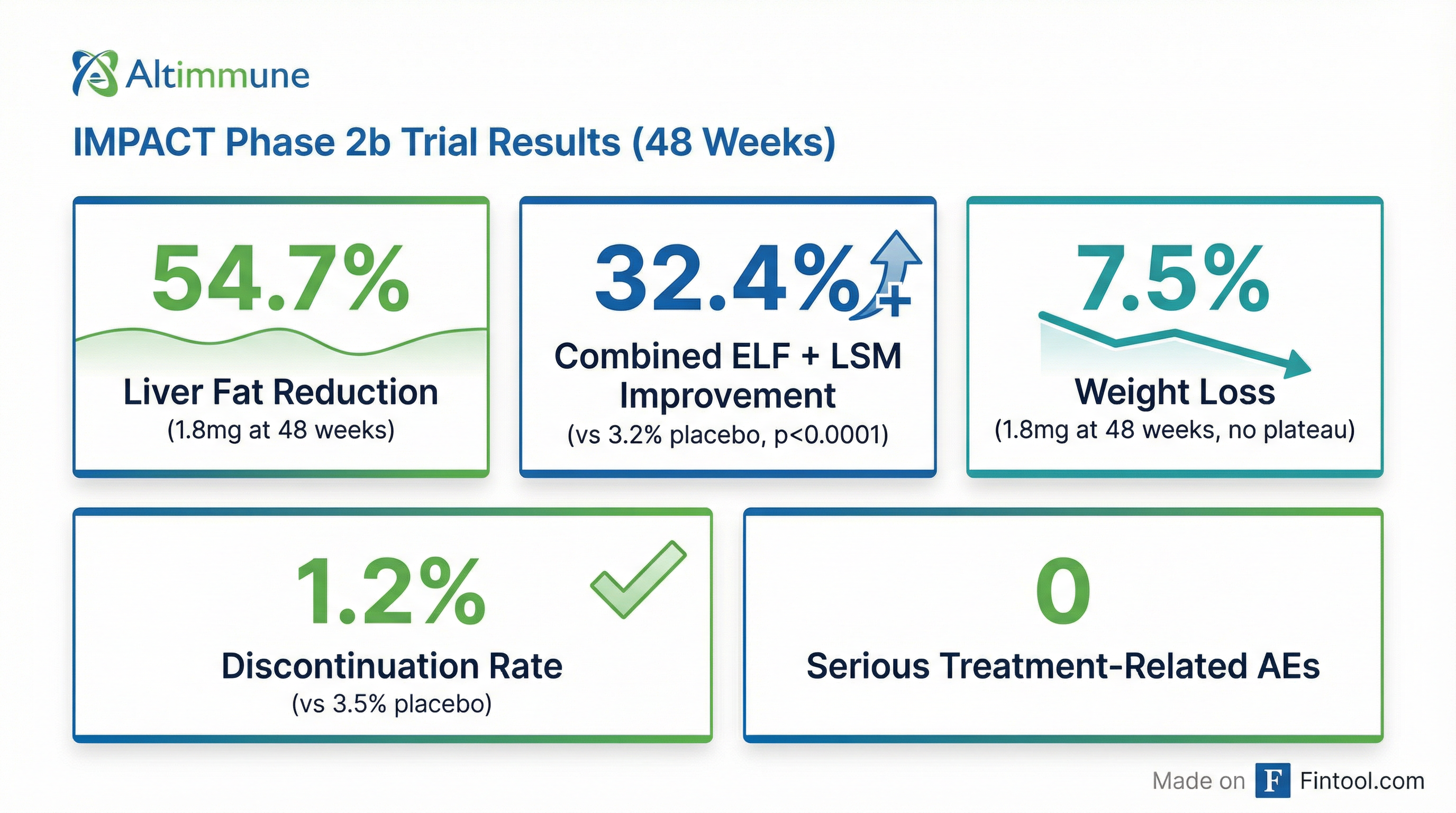
The Clinical Case: 48-Week IMPACT Data
The full 48-week data from the IMPACT trial, released in December 2025, paint a compelling picture for pemvidutide's efficacy.
| Metric | Result | Significance |
|---|---|---|
| ELF Score Reduction | Statistically significant | Key non-invasive fibrosis marker |
| Liver Stiffness (LSM) | Statistically significant reduction | Validated fibrosis assessment |
| Weight Loss (1.8mg) | 7.5% | No plateau at 48 weeks |
| Discontinuation Rate (AEs) | 1.2% vs 3.5% placebo | Superior tolerability |
Notably, the weight loss trajectory showed no evidence of plateauing at 48 weeks, suggesting continued metabolic benefits with longer treatment duration—a critical differentiator in a market where sustained efficacy is paramount.
The Mechanism Advantage: Why 1:1 Matters
Pemvidutide's scientific differentiation lies in its balanced 1:1 glucagon/GLP-1 dual receptor agonist design.
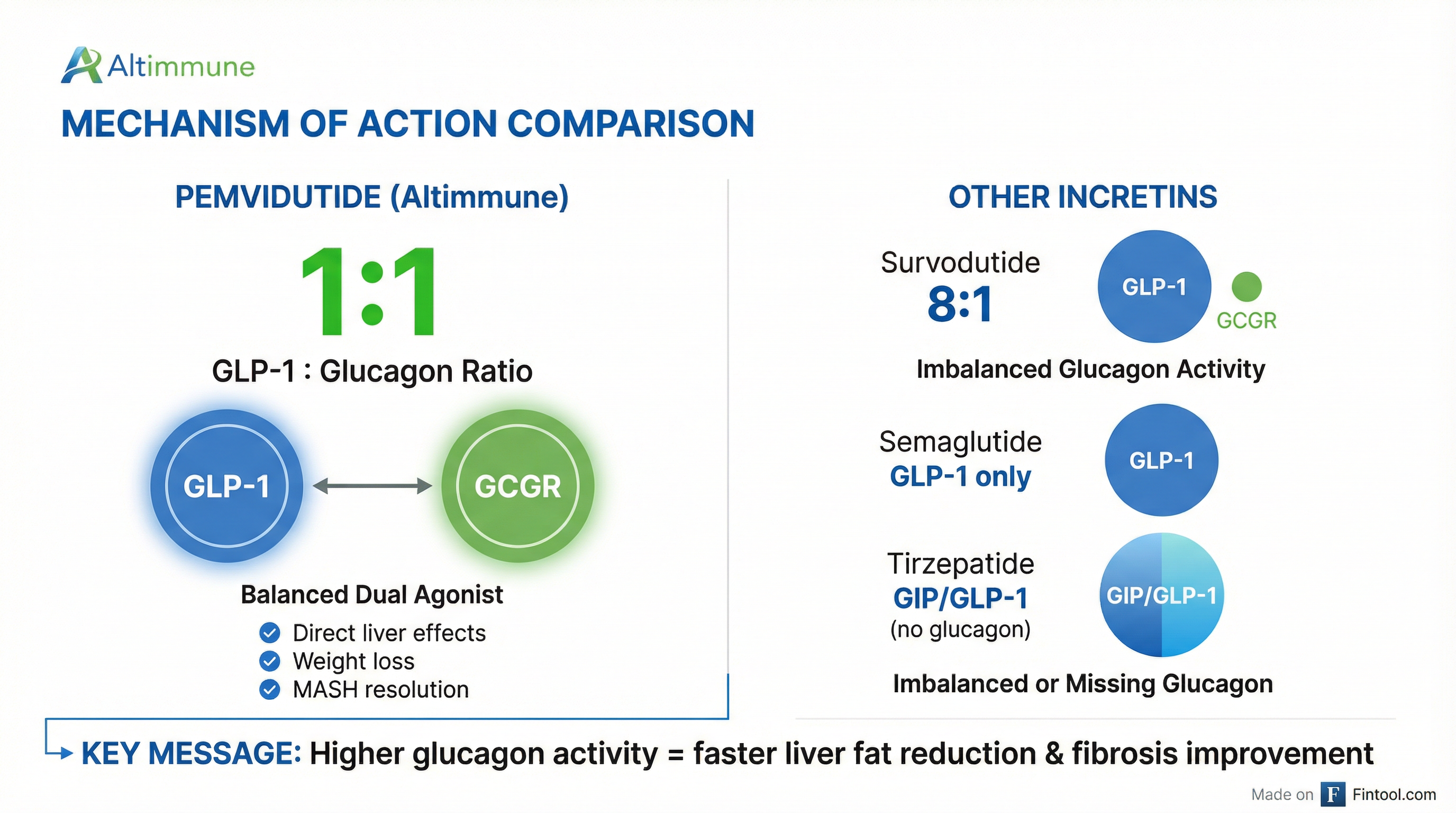
While GLP-1 agonists like Novo Nordisk's semaglutide and Eli Lilly's tirzepatide have dominated obesity headlines, MASH requires a different approach. The disease fundamentally involves liver fat accumulation and fibrosis—and that's where glucagon's direct hepatic effects become critical.
Altimmune's Chief Commercial Officer Mitchel Sayare has emphasized this distinction: pemvidutide is designed as a "complete solution" that combines direct liver fat reduction from the glucagon component with the metabolic benefits of GLP-1 signaling.
Management has directly contrasted this with Boehringer Ingelheim's survodutide, which uses an 8:1 GLP-1 to glucagon ratio. Altimmune argues that the higher glucagon activity in pemvidutide's balanced 1:1 formulation provides more pronounced direct effects on the liver—precisely where MASH patients need it most.
The $30 Billion Market Opportunity
The MASH market represents one of the largest untapped opportunities in pharmaceutical development. According to Madrigal's filings, approximately 525,000 U.S. patients have MASH with moderate to advanced fibrosis (F2-F3)—the population most urgently in need of treatment.
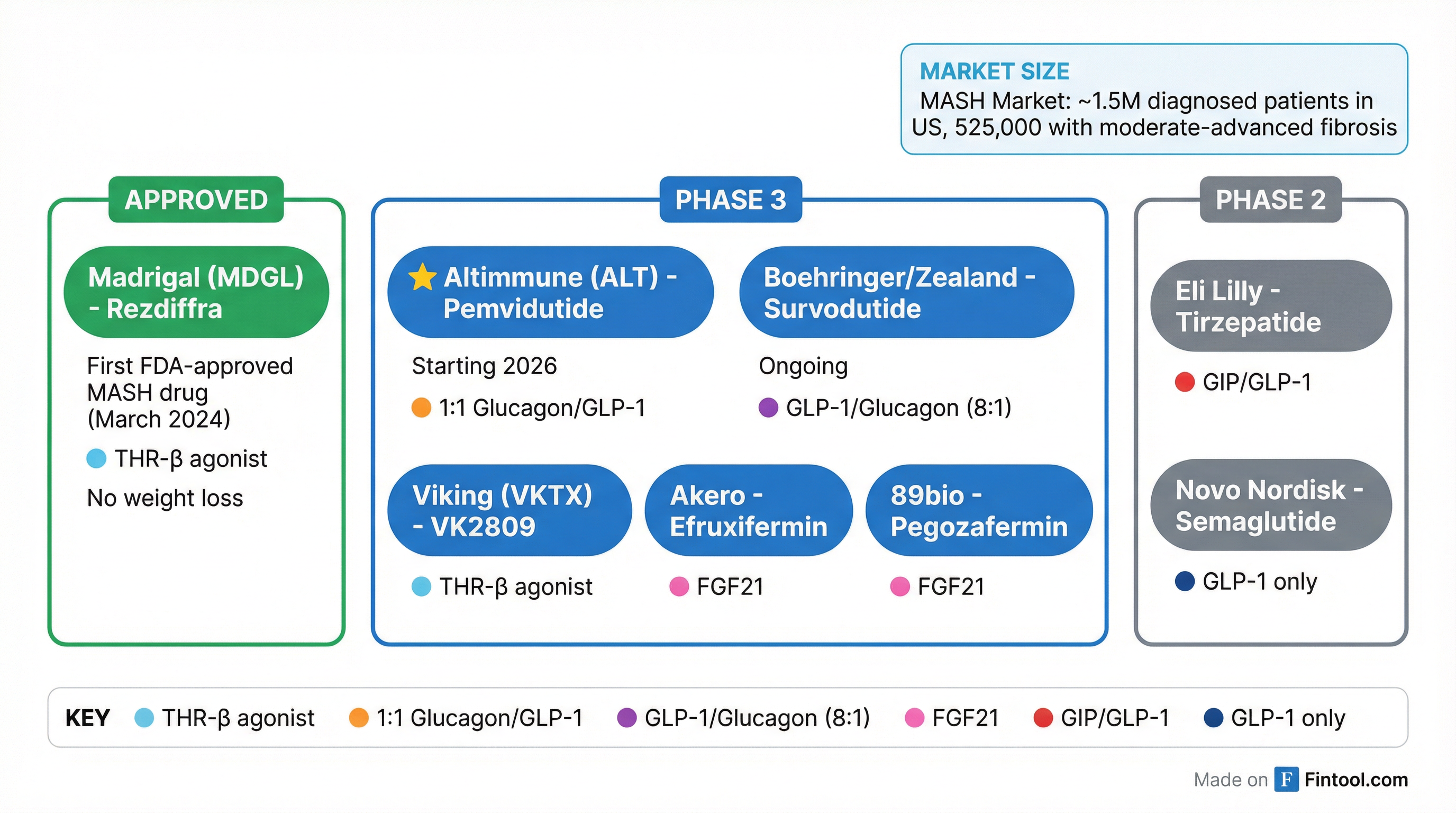
The competitive landscape is intensifying:
| Company | Drug | Mechanism | Status |
|---|---|---|---|
| Madrigal | Rezdiffra | THR-β agonist | Approved (March 2024) |
| Altimmune | Pemvidutide | GLP-1/Glucagon (1:1) | Phase 3 planned 2026 |
| Viking Therapeutics | VK2809 | THR-β agonist | Phase 3 |
| Boehringer/Eli Lilly | Survodutide | GLP-1/Glucagon (8:1) | Phase 3 |
| Novo Nordisk | Semaglutide | GLP-1 | Phase 3 (MASH indication) |
Altimmune's management has been explicit about their positioning against Rezdiffra, which lacks the weight loss component that pemvidutide delivers. In a market where obesity and MASH frequently co-occur, the ability to address both conditions simultaneously could prove decisive.
Financial Position: Runway for Phase 3
As a clinical-stage biotech, Altimmune remains pre-revenue, posting a net loss of $19.0 million for Q3 2025.
However, the company maintains a solid cash position. As of the end of Q3 2025, Altimmune reported approximately $211 million in cash, cash equivalents, and marketable securities—providing runway to initiate and execute the planned Phase 3 program.
| Metric | Q3 2025 |
|---|---|
| Cash Position | $211M |
| Net Loss | -$19.0M |
| Revenue | Pre-revenue stage |
The path to Phase 3 is now clear. Altimmune has aligned with the FDA on a registrational Phase 3 trial design, planned for initiation in 2026. The 52-week trial will incorporate biopsy-based endpoints that could support accelerated approval—a regulatory pathway that becomes significantly more accessible with Breakthrough Therapy Designation.
Market Reaction and Valuation
The 15% after-hours pop reflects investor enthusiasm, but ALT remains deeply discounted relative to analyst expectations. The consensus price target of $17.75 implies more than 300% upside from current levels—suggesting the Street sees significant option value in pemvidutide's Phase 3 prospects.
For context, Altimmune's current market cap of roughly $400 million pales in comparison to the multi-billion dollar valuations of MASH competitors:
- Madrigal (MDGL): ~$6 billion market cap with Rezdiffra already generating revenue
- Viking (VKTX): ~$5 billion market cap on Phase 3 THR-β program
The valuation gap underscores both the risk—pemvidutide must still clear Phase 3—and the potential reward if the drug ultimately reaches market.
What to Watch
Near-term catalysts:
- Phase 3 trial initiation (expected 2026)
- Additional pemvidutide data presentations at medical conferences
- Partnership discussions—management has indicated openness to strategic collaborations
Key risks:
- Phase 3 execution and efficacy confirmation
- Cash runway relative to trial costs
- Competitive dynamics as multiple MASH drugs advance
- Regulatory standards for accelerated approval
The bigger picture: Today's Breakthrough Therapy Designation doesn't guarantee success, but it materially de-risks the regulatory path. For a company that was trading at $3.61 as recently as this morning, the FDA's validation of pemvidutide's potential represents a significant inflection point.