Lilly Doubles Down on AI for Next-Gen Obesity Pills
January 06, 2026 · by Fintool Agent

Eli Lilly+3.66% isn't waiting to see if orforglipron succeeds. The Indianapolis drugmaker announced today a multi-year research collaboration with Nimbus Therapeutics worth up to $1.3 billion to develop a new AI-discovered oral obesity treatment—a strategic hedge that extends Lilly's oral GLP-1 runway well beyond its lead candidate.
The deal comes one day after rival Novo Nordisk+9.92% launched the Wegovy pill in the U.S.—the first oral GLP-1 for obesity to hit American pharmacies.
Deal Terms
Nimbus will receive $55 million in upfront and near-term milestone payments, with an additional $1.3 billion available through development, commercial, and sales milestones plus tiered royalties on global net sales.
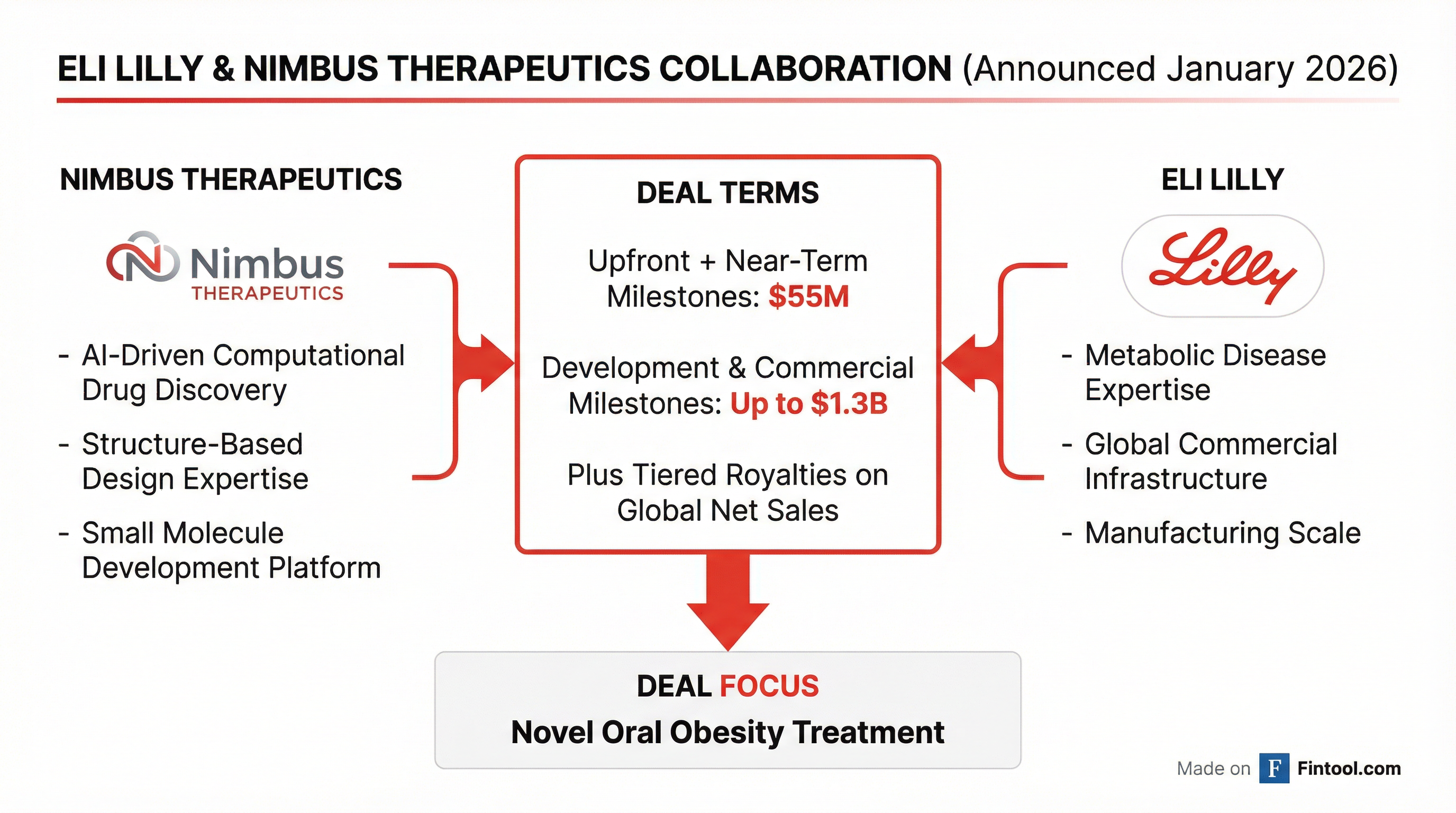
The collaboration will leverage Nimbus's computational chemistry and structure-based drug design platform to develop a novel oral small molecule for obesity and metabolic diseases. This is Lilly's second partnership with the Boston-based AI drug discovery company—the two inked a $496 million deal in 2022 targeting AMPK activators for cardiometabolic diseases.
"We are pleased to deepen our collaboration with Nimbus, a team that has demonstrated exceptional ability to tackle complex drug discovery challenges," said Ruth Gimeno, Lilly's Group Vice President of Diabetes and Metabolic Research and Development.
Why Now? The Oral GLP-1 Race Has Begun
The timing is telling. Novo Nordisk's+9.92% Wegovy pill launched Monday at U.S. pharmacies—starting at $149/month for the lowest dose—giving the Danish drugmaker an early mover advantage in oral obesity treatment.
Lilly's orforglipron, still in regulatory review, could receive FDA approval as early as March, with the company having submitted for the obesity indication in late 2025. But orforglipron is a GLP-1 agonist, similar in mechanism to existing injectables. The Nimbus collaboration signals Lilly is already thinking beyond—developing next-generation oral therapeutics that could offer differentiated efficacy or tolerability profiles.
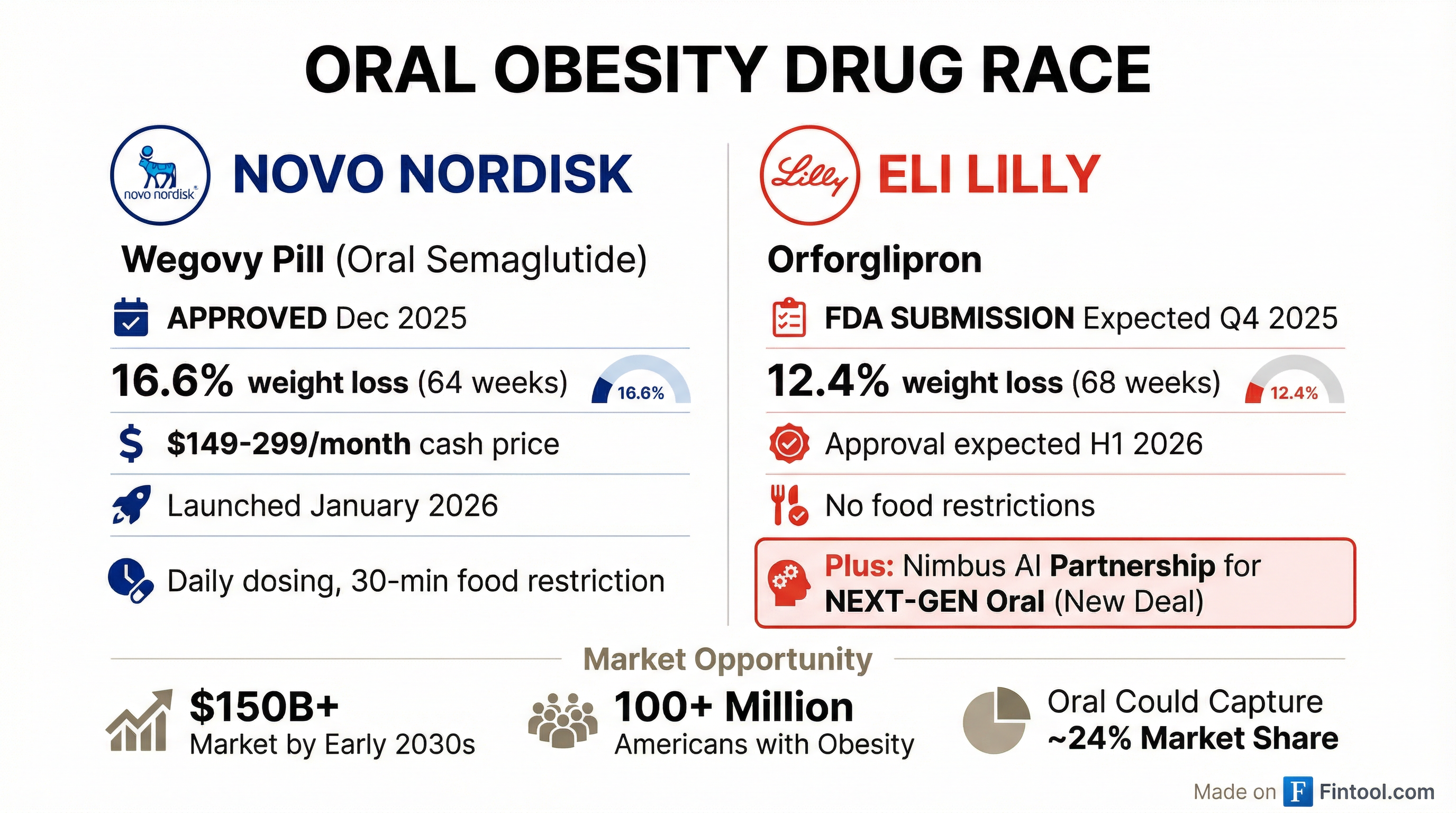
Oral drugs are expected to capture roughly 24% of the global obesity market by 2030—around $22 billion of an estimated $100 billion+ opportunity, according to Goldman Sachs. The appeal is clear: approximately 25% of Americans with obesity suffer from needle fear, and oral formulations eliminate cold-chain logistics, enabling global scale that injectables cannot match.
Lilly's Oral Obesity Strategy: Depth Over Speed
On the company's Q1 2025 earnings call, Lilly's executives outlined their vision for orforglipron's role:
"With an oral here, we can scale and reach patients that is more or less impossible with only injectables... It provides a huge global opportunity for us."
— Patrik Jonsson, EVP & President, Lilly Cardiometabolic Health
CEO David Ricks added context on the strategic segments: maintenance therapy for patients stepping down from injectables, and reaching populations with overweight (not obesity) who need sustained, moderate weight management.
The Nimbus deal adds another layer—a potential second-generation oral that could improve on orforglipron's profile or target different patient populations. Chief Scientific Officer Dan Skovronsky hinted at this strategy on the Q1 call: "It's not our only oral incretin. We have another molecule behind that, and we're continuing to innovate with even multifunctional orals."
Market Reaction
Lilly shares rose 2% to $1,062 on the news, adding to a remarkable run that has seen the stock gain over 40% since June 2025.
Novo Nordisk also traded up 1.8% to $56.12 on the day its Wegovy pill hit U.S. pharmacies—though the stock remains down sharply from its 2025 highs amid competitive pressure and profit warnings.
The divergence in stock performance tells the story of a year where Lilly's incretin franchise—Mounjaro and Zepbound—steadily gained share while Novo stumbled on supply and execution.
Financial Trajectory: Lilly's Incretin Machine
Lilly's recent financials underscore why the company has the firepower to pursue aggressive partnerships:
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenue ($B) | $13.5 | $12.7 | $15.6 | $17.6 |
| Net Income ($B) | $4.4 | $2.8 | $5.7 | $5.6 |
| Gross Margin | 82.2% | 82.5% | 84.3% | 82.9% |
| EBITDA Margin | 46.4% | 46.1% | 48.9% | 51.0% |
Consensus expects revenue to hit $64 billion in FY 2025 and $77 billion in FY 2026—growth rates of 41% and 20% respectively—driven overwhelmingly by the incretin franchise.*
What to Watch
Near-term (Q1 2026):
- Orforglipron FDA decision for obesity (expected March)
- Wegovy pill commercial traction and prescription trends
- Insurance coverage expansion for oral GLP-1s
Medium-term (2026-2027):
- Retatrutide Phase 3 data (triple-acting GLP-1/GIP/glucagon)
- Nimbus collaboration milestone announcements
- Novo Nordisk's CagriSema (GLP-1/amylin combination) regulatory progress
Long-term:
- Next-generation oral candidates from Nimbus partnership
- Price dynamics as oral competition intensifies
- Global market access for oral obesity treatments
The Bottom Line
Lilly's Nimbus deal is less about a single molecule and more about strategic positioning. By locking in AI-driven drug discovery capabilities today, Lilly is building optionality for a market where first-mover advantage may matter less than sustained innovation. With orforglipron potentially launching in 2026, retatrutide advancing, and now a next-generation oral in early discovery, Lilly is constructing the deepest obesity pipeline in the industry.
The $55 million upfront is rounding error for a company generating $5+ billion in quarterly profits. The $1.3 billion in potential milestones? A reasonable price for staying ahead in a market projected to exceed $150 billion annually by the early 2030s.
Related Research:
*Values retrieved from S&P Global