Eli Lilly Bets $1.12 Billion on Next-Gen Gene Editing for Hearing Loss
January 28, 2026 · by Fintool Agent

Eli Lilly+3.66% is doubling down on genetic medicine with a deal worth up to $1.12 billion to license Germany-based Seamless Therapeutics' next-generation gene editing platform for hearing loss treatments.
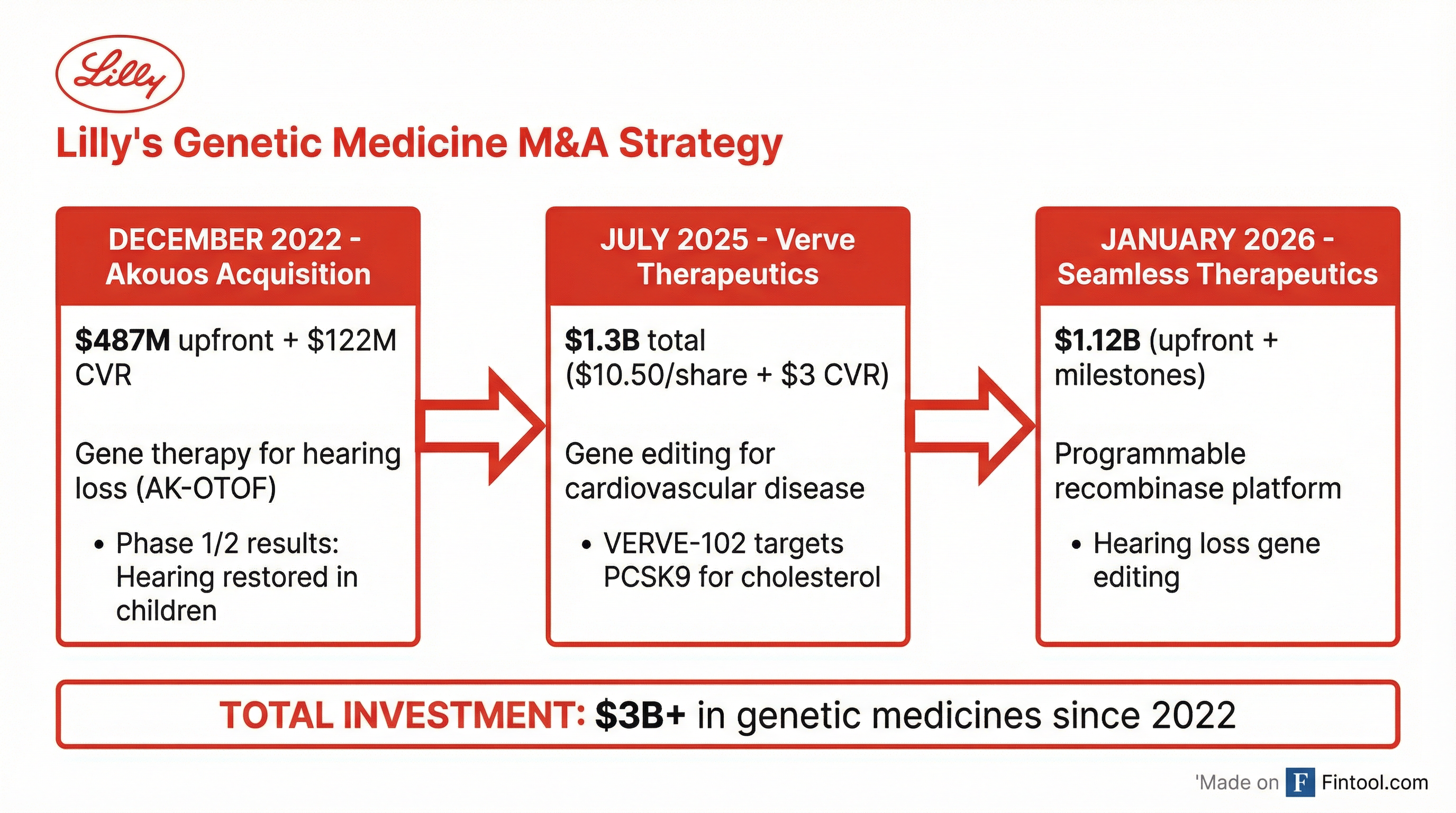
The partnership marks Lilly's third major genetic medicine transaction since 2022 and brings its total investment in the space to over $3 billion—a clear signal that the Indianapolis-based pharma giant sees gene-based treatments as a critical growth engine beyond its blockbuster obesity and diabetes franchises.
Lilly shares closed at $1,023.80, down 1.5% on the day, giving the company a market capitalization of approximately $918 billion.
Deal Structure
Seamless will receive a combination of an upfront payment and R&D funding, with additional payments tied to development and commercial milestones totaling more than $1.12 billion. The company will also be eligible for tiered royalties on any approved products.
Under the agreement, Seamless will design and program site-specific recombinases directed at correcting mutations in genes linked to hearing loss. Lilly will then receive an exclusive license to advance these therapies through preclinical testing and commercialization.
"This collaboration is a validation of our gene editing platform and its broad disease-modifying potential," said Albert Seymour, Seamless CEO. "We look forward to working with our partners at Lilly in our shared goal to transform the outcome for patients with genetic hearing loss."
Why Recombinases Matter
Seamless's platform represents a meaningful technological advancement over first-generation gene editing approaches like CRISPR. Traditional CRISPR systems create double-strand DNA breaks and rely on the cell's natural repair pathway—a process that can introduce insertions or deletions at unintended locations.
Recombinases, by contrast, are enzymes that cut and rejoin DNA directly at specific sequences, using a dual targeting mechanism that boosts specificity. According to Seamless, this approach "all but eliminates off-target activity."
"Recombinases have been used in scientific research for decades, but only now are they being harnessed for the potential of commercialized therapies," noted Pharmaceutical Technology in its coverage of the deal. The platform can perform large but precise DNA edits along the genome, theoretically enabling treatment across a broad range of genetic diseases.
Lilly's Hearing Loss Strategy
The Seamless deal expands a hearing loss portfolio that Lilly began building with its 2022 acquisition of Akouos for $487 million upfront plus a $122 million contingent value right. Through Akouos, Lilly acquired gene therapies targeting several genetic forms of hearing loss, including:
- GJB2 (connexin 26) for the most common form of monogenic deafness
- AK-OTOF for hearing loss due to otoferlin gene mutations
- AK-CLRN1 for Usher Type 3A (progressive hearing and vision loss)
- AK-antiVEGF for vestibular schwannoma
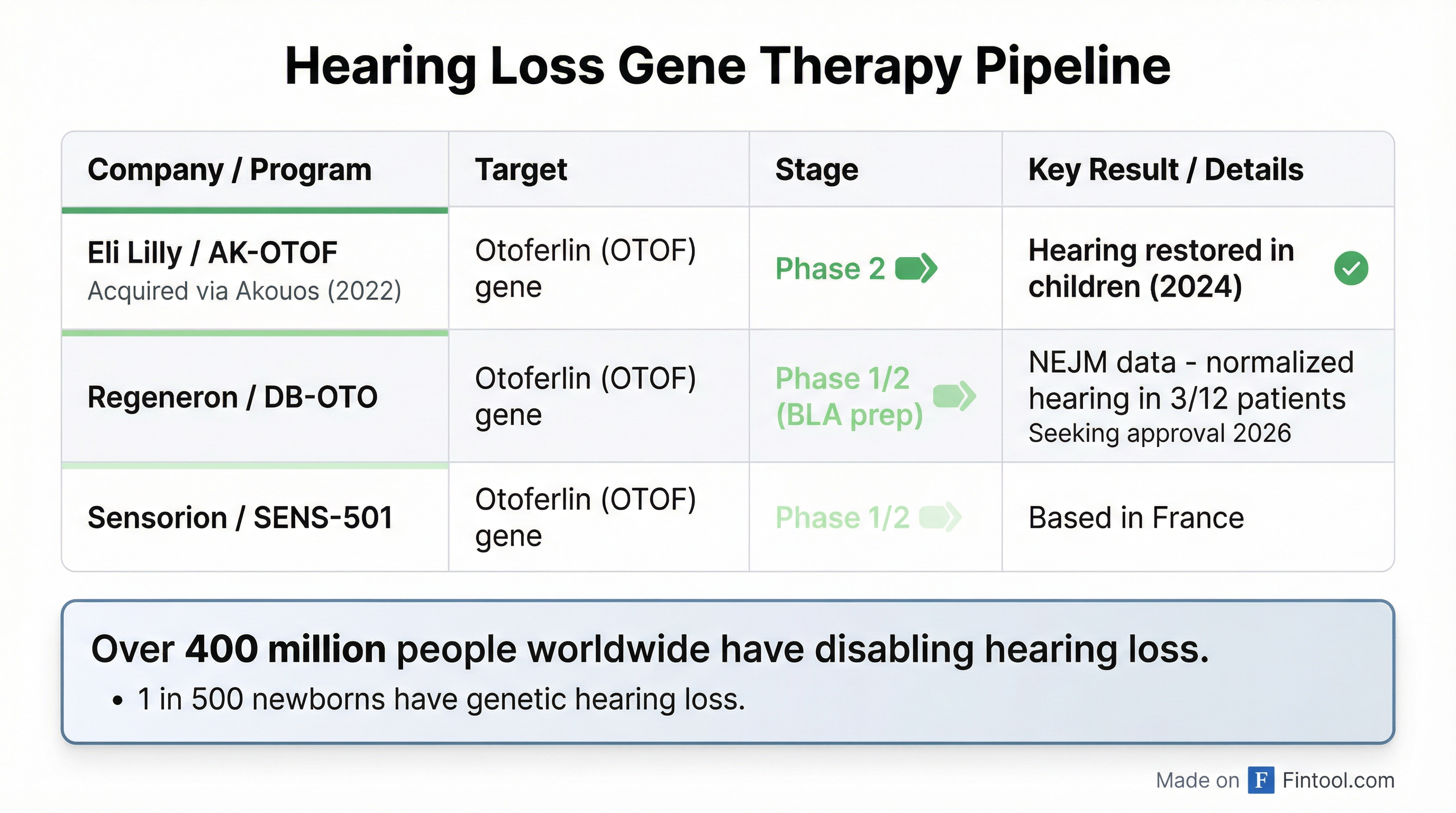
The AK-OTOF program has already demonstrated clinical proof of concept. In January 2024, Lilly announced "hearing restoration within 30 days of a single administration in the first participant, an individual with more than a decade of profound hearing loss." The program has since advanced to Phase 2.
The company has also partnered with South Korea-based Rznomics for RNA-based hearing loss therapies, further deepening its commitment to the space.
Competitive Landscape
Lilly is not alone in pursuing gene therapies for hearing loss. Regeneron+2.46% has emerged as the most advanced competitor with its DB-OTO program, which targets the same otoferlin gene mutations as Lilly's Akouos-derived therapy.
In October 2025, Regeneron published "game-changing" Phase 1/2 data in The New England Journal of Medicine showing DB-OTO significantly improved hearing in nearly a dozen children with congenital deafness. Three of 12 treated patients achieved normalized hearing sensitivity.
"What we're talking about, really, is: what's the value of hearing?" said a Regeneron executive. "My expectation is most people see massive value, having hearing connect you to your environment, to others, to your families."
Regeneron plans to seek FDA approval for DB-OTO in 2026, potentially making it the first approved gene therapy for genetic hearing loss. The likely multi-million dollar price tag for gene therapies will test payer appetite, though the one-time treatment model offers significant value versus lifetime cochlear implant costs.
France-based Sensorion also has a Phase 1/2 program (SENS-501) targeting otoferlin mutations.
$3 Billion Bet on Genetic Medicine
The Seamless partnership fits a clear pattern of Lilly using its balance sheet to build a diversified genetic medicine portfolio. Beyond hearing loss, the company acquired Verve Therapeutics in July 2025 for approximately $1.3 billion ($10.50 per share plus up to $3.00 in contingent value rights).
Verve brings gene editing medicines for cardiovascular disease, including VERVE-102, which targets PCSK9—a gene linked to cholesterol levels. The program has FDA Fast Track designation and is being evaluated in a Phase 1b clinical trial.
"Through the acquisition of Verve Therapeutics, we added several genetic medicines for cardiovascular disease that may only need to be given once in a lifetime," said Daniel Skovronsky, Lilly's Chief Scientific Officer, on the Q2 2025 earnings call.
| Deal | Date | Value | Focus |
|---|---|---|---|
| Akouos | Dec 2022 | $487M + $122M CVR | Hearing loss gene therapy |
| Verve Therapeutics | Jul 2025 | $1.3B | Cardiovascular gene editing |
| Seamless Therapeutics | Jan 2026 | $1.12B | Recombinase platform for hearing loss |
| Total | $3B+ |
Lilly told Reuters that the deal "reflects our sustained investment in genetic medicines, an area where platform technologies can address diseases with significant unmet need that are difficult or impossible to treat with traditional approaches."
Financial Position Supports Growth
Lilly's aggressive dealmaking is backed by substantial financial firepower. The company generated $17.6 billion in revenue in Q3 2025, up from $11.4 billion in the year-ago quarter—driven primarily by the explosive growth of Mounjaro and Zepbound.
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenue | $13.5B | $12.7B | $15.6B | $17.6B |
| Net Income | $4.4B | $2.8B | $5.7B | $5.6B |
| Cash & Equivalents | $3.3B | $3.1B | $3.4B | $9.8B |
The company has been ramping capital expenditure to support manufacturing expansion, spending $2.1 billion in Q3 2025 alone. CEO David Ricks noted on the Q2 2025 call that Lilly "produced more than 1.6 times the amount of saleable incretin doses during 2025 when compared to 2024."
What to Watch
Near-term catalysts:
- Seamless advancing recombinases to lab studies by end of 2026
- Lilly's AK-OTOF Phase 2 data readouts
- Regeneron's potential FDA filing for DB-OTO
Key questions:
- Can recombinase technology achieve the precision advantages claimed over CRISPR in clinical settings?
- Will payers accept multi-million dollar pricing for hearing loss gene therapies?
- How will Lilly differentiate its hearing loss pipeline from Regeneron's lead?
The Seamless deal underscores Lilly's strategic pivot toward genetic medicines as a complement to its cardiometabolic portfolio. With over $3 billion deployed since 2022, the company is making a clear bet that one-time treatments for genetic diseases represent the next frontier of pharmaceutical innovation.
Related: Eli Lilly+3.66% · Regeneron+2.46%