Lilly's Zepbound-Taltz Combo Delivers 40x Response Rate in First-of-Its-Kind Arthritis-Obesity Trial
January 8, 2026 · by Fintool Agent
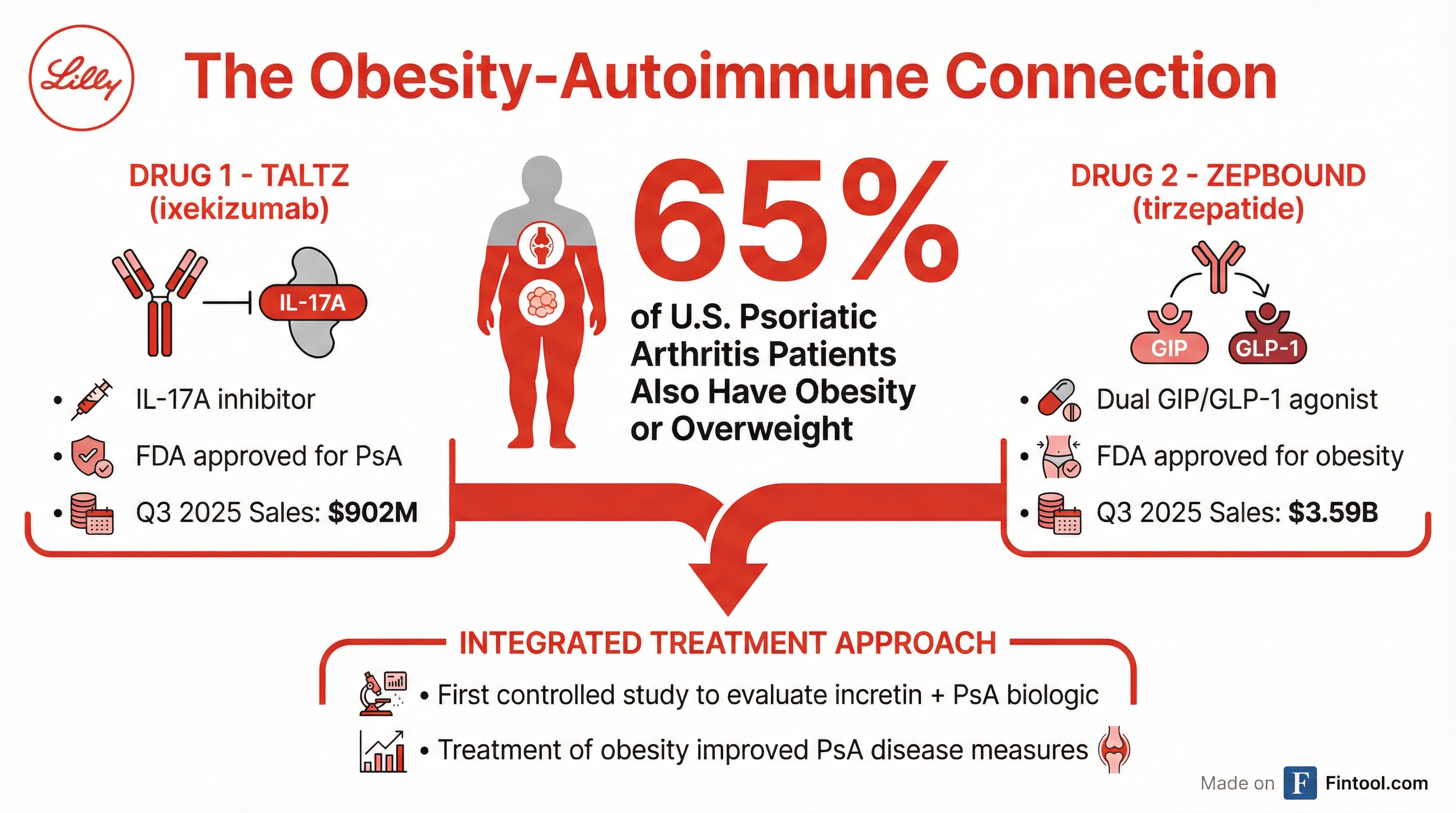
Eli Lilly+3.66% announced today that its combination of Zepbound and Taltz delivered overwhelming efficacy in the TOGETHER-PsA trial—the first controlled study to evaluate an incretin therapy alongside a psoriatic arthritis biologic. Patients receiving both drugs achieved the primary endpoint at a rate 40 times higher than those on Taltz alone, signaling a potential paradigm shift in how physicians treat the estimated 65% of psoriatic arthritis patients who also suffer from obesity.
The results reinforce Lilly's dominant position in metabolic disease and suggest the company's incretin franchise may have broader therapeutic applications than previously priced into the stock, which traded down 3.2% to $1,073 on a broader tech selloff despite the positive data.
The Numbers That Matter
At 36 weeks, 31.7% of patients receiving Taltz plus Zepbound achieved the composite primary endpoint—defined as at least 50% improvement in psoriatic arthritis activity (ACR50) combined with at least 10% weight loss. Only 0.8% of patients on Taltz monotherapy hit that bar.
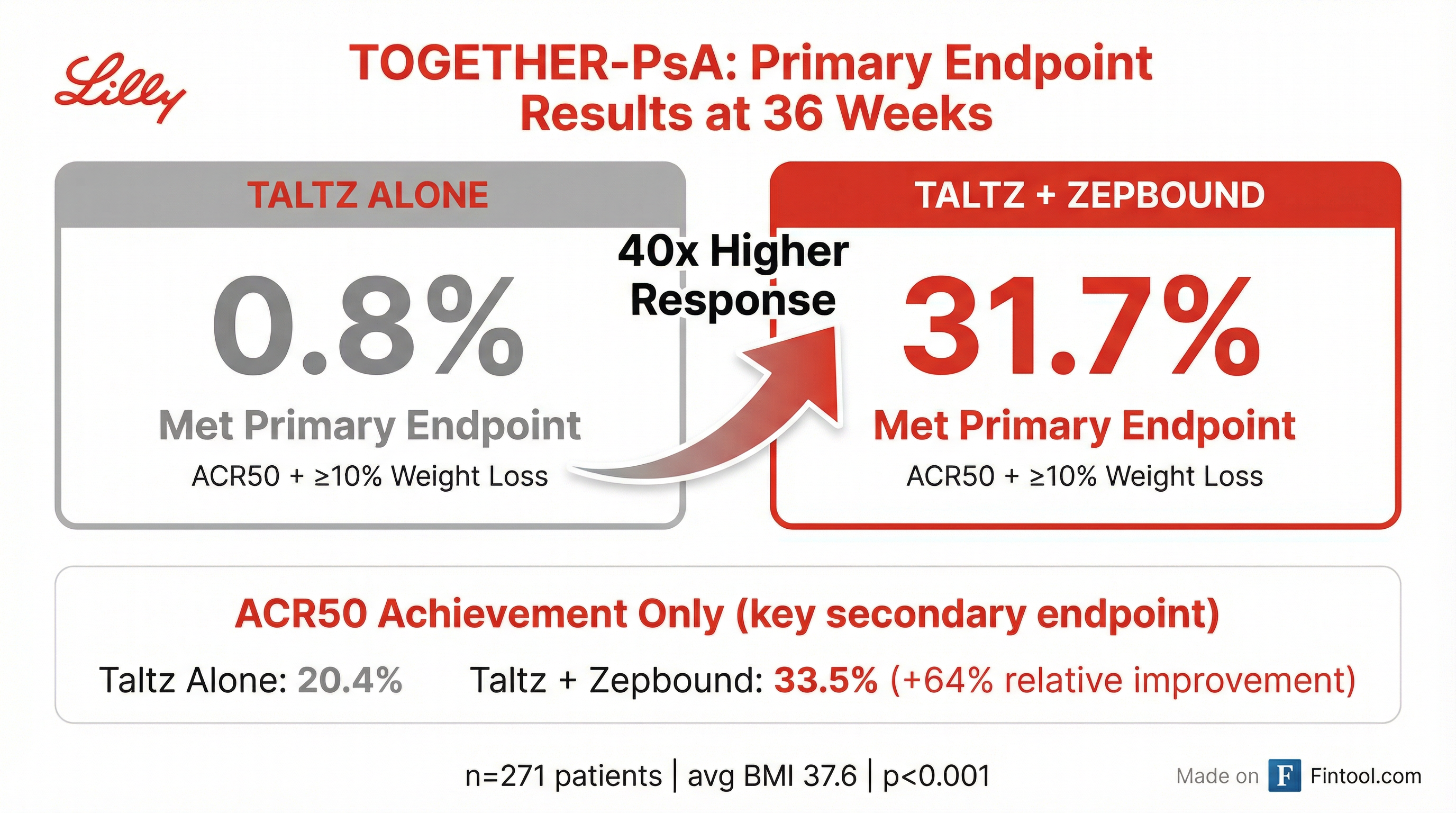
Perhaps more striking: even on the arthritis measure alone (ACR50), the combination therapy outperformed. Some 33.5% of patients on both drugs achieved ACR50 versus 20.4% on Taltz alone—a 64% relative improvement. This suggests that treating obesity with Zepbound doesn't just help patients lose weight; it actually reduces the burden of their autoimmune disease.
"These results demonstrate how an integrated treatment approach has the potential to improve the standard of care in a compelling and comprehensive way," said Mark Genovese, senior vice president of Lilly Immunology Development.
Why This Trial Is Different
The TOGETHER-PsA study wasn't just testing whether two drugs work better than one. It was testing a hypothesis: that obesity isn't merely a comorbidity for autoimmune patients—it's a disease modifier. The data appear to confirm this.
The 271-patient trial enrolled adults with active psoriatic arthritis who were either obese (BMI ≥30) or overweight (BMI 27-29.9) with at least one weight-related condition. The average BMI was 37.6 across both arms. Critically, more than 60% of participants had prior experience with one or more advanced therapies—a difficult-to-treat population that often fails standard treatment.
Dr. Joseph Merola of UT Southwestern Medical Center, who was involved in the trial, called the findings a "potential paradigm shift." He noted: "The observed benefit with treatment using Taltz and Zepbound appears to meaningfully impact psoriatic disease activity, indicating that for many patients, PsA is an obesity-related condition."
Financial Context: The Lilly Juggernaut
This trial arrives as Lilly's cardiometabolic franchise continues its explosive growth trajectory. In Q3 2025, Zepbound generated $3.59 billion in revenue—up 185% year-over-year—while Mounjaro added another $6.52 billion. Together, these two tirzepatide-based drugs now represent nearly 60% of Lilly's total sales.
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Total Revenue ($B) | $13.5 | $12.7 | $15.6 | $17.6 |
| Zepbound Revenue ($B) | $1.26 | $2.31 | $3.38 | $3.59 |
| Mounjaro Revenue ($B) | $3.11 | $3.84 | $5.20 | $6.52 |
| Taltz Revenue ($M) | $880 | $762 | $848 | $902 |
| Gross Margin % | 82.2% | 82.5% | 84.3% | 82.9% |
Taltz, the IL-17A inhibitor that forms the other half of this combination, is a mature but stable asset generating roughly $3.5 billion in annualized revenue across immunology indications including psoriatic arthritis, plaque psoriasis, and ankylosing spondylitis.
The Competitive Angle
The TOGETHER-PsA results have implications beyond Lilly. Novo Nordisk+9.92%, which trails Lilly in U.S. obesity market share, has not announced comparable trials combining its Wegovy or Ozempic with autoimmune biologics. If treating obesity is shown to improve outcomes across multiple autoimmune conditions—not just psoriatic arthritis—Lilly's first-mover advantage in this approach could widen.
Lilly has already announced that topline results from a parallel trial, TOGETHER-PsO, are expected in the first half of 2026. That study examines Zepbound plus Taltz in patients with moderate-to-severe plaque psoriasis and obesity.
Separately, Lilly is awaiting a Q1 2026 FDA decision on orforglipron, its oral GLP-1 candidate. Approval would give Lilly a potential first-in-class oral obesity pill, though recent data showed somewhat elevated discontinuation rates.
Safety Profile
Adverse events in the combination arm were described as generally mild to moderate and consistent with the known safety profiles of each drug. The most common side effects included nausea, diarrhea, constipation, and injection site reactions—all familiar to GLP-1 class users.
What to Watch
Near-term catalysts:
- Detailed 36-week results presentation at an upcoming medical meeting (date TBD)
- Regulatory discussions on potential labeling expansion
- TOGETHER-PsO topline data (H1 2026)
- Orforglipron FDA decision (Q1 2026)
Longer-term questions:
- Will payors cover combination therapy at current prices?
- Can this data support a psoriatic arthritis indication for Zepbound alone?
- What other autoimmune conditions might benefit from incretin therapy?
The Bottom Line
Lilly just demonstrated that its obesity blockbuster might be an immunology drug too. The TOGETHER-PsA data—showing treatment of obesity materially improves autoimmune disease outcomes—could open new indications, new prescribers, and new reimbursement pathways for a drug already generating $14 billion in annualized sales. For Novo Nordisk, the pressure to demonstrate similar combination efficacy just intensified.
Related: