Patient Deaths Halt Merck's $5.5 Billion ADC Trial: Second Major Blow to Daiichi Partnership
December 19, 2025 · by Fintool Agent

The FDA has placed a partial clinical hold on Merck's Phase 3 trial of ifinatamab deruxtecan (I-DXd) after an undisclosed number of patient deaths from interstitial lung disease (ILD), dealing a second major setback to its $5.5 billion antibody-drug conjugate partnership with Japan's Daiichi Sankyo.
The IDeate-Lung02 trial had enrolled over 500 patients with relapsed small cell lung cancer—one of the deadliest and most treatment-resistant cancers—and was the cornerstone of Merck's hopes for the first B7-H3 directed ADC. The companies have not disclosed how many patients died.
A Pattern of Trouble
This marks the second major failure for Merck's landmark ADC deal with Daiichi Sankyo. Earlier this year, the partners pulled their FDA filing for patritumab deruxtecan (HER3-DXd) after a confirmatory trial failed to show an overall survival benefit and revealed treatment-related deaths, including some from ILD.
Of the three ADC candidates Merck licensed for $5.5 billion in upfront and near-term payments in October 2023, two have now hit critical roadblocks:
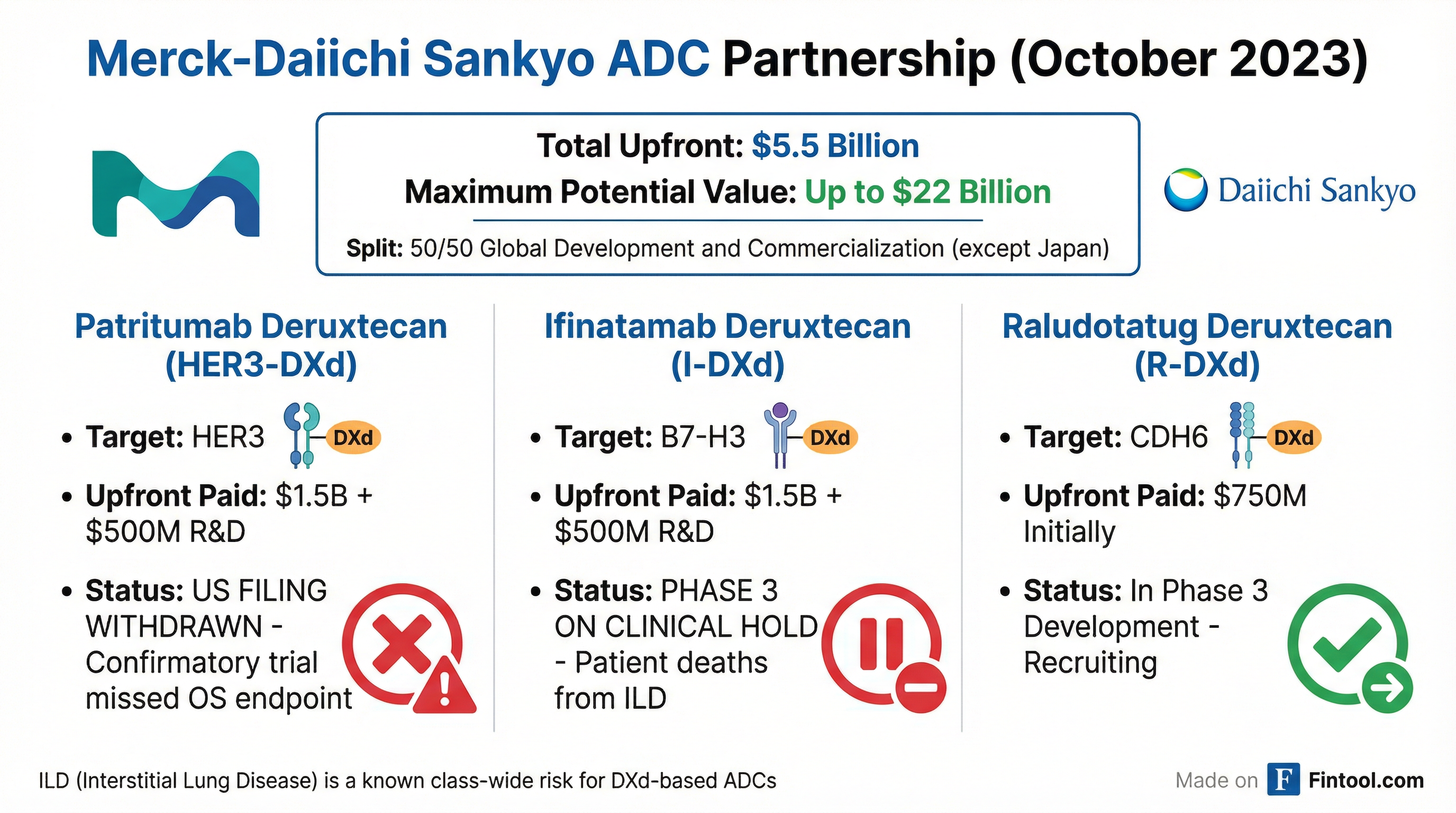
| ADC Candidate | Target | Upfront Investment | Current Status |
|---|---|---|---|
| Patritumab deruxtecan | HER3 | $2B ($1.5B + $500M R&D) | US filing withdrawn |
| Ifinatamab deruxtecan | B7-H3 | $2B ($1.5B + $500M R&D) | Phase 3 on clinical hold |
| Raludotatug deruxtecan | CDH6 | $750M (with $750M continuation) | Still in development |
The deal terms specified Merck would pay Daiichi Sankyo $4 billion upfront plus $1.5 billion in continuation payments, with potential milestone payments reaching up to $22 billion if all three drugs succeed.
The ILD Problem Is Class-Wide
Interstitial lung disease—a potentially fatal condition where lung tissue becomes scarred and inflamed—has emerged as a defining safety challenge for Daiichi Sankyo's entire deruxtecan-based ADC platform. The company's technology links antibodies to a topoisomerase I inhibitor payload (DXd) using tetrapeptide-based cleavable linkers. While highly effective at killing cancer cells, this mechanism appears to carry inherent lung toxicity risks.
The problem extends beyond Merck's partnership:
-
Enhertu (Astrazeneca-Daiichi partnership): The HER2-directed ADC carries a boxed warning for ILD and pneumonitis. Fatal outcomes have occurred.
-
Datroway (Astrazeneca-Daiichi partnership): The TROP2-directed ADC approved in January 2025 has an ILD warning. Additionally, the Tropion-Lung15 trial has been temporarily halted in EU countries due to "an increase in overall cases of high-grade ILD."
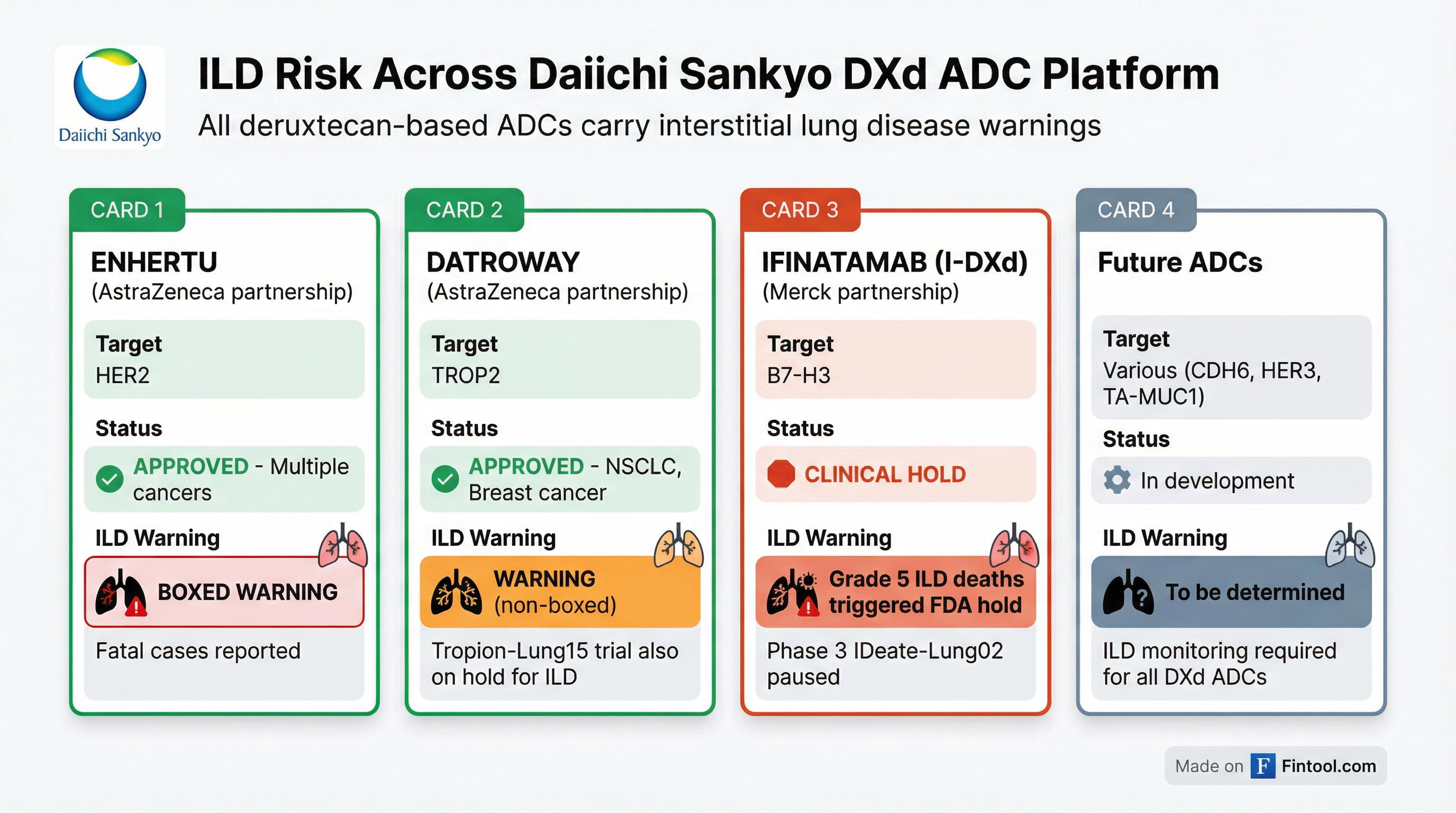
"ILD is a potentially fatal side effect known to be associated with all of Daiichi's DX ADCs," Jefferies analysts noted. "This has not stopped Enhertu and Datroway from being approved."
Devastating for Small Cell Lung Cancer Patients
The clinical hold is particularly cruel for patients with relapsed small cell lung cancer, who have virtually no effective treatment options. SCLC represents approximately 15% of all lung cancers with over 200,000 cases diagnosed globally each year. The disease is characterized by rapid growth and early metastatic spread—approximately 70-80% of patients present with extensive-stage disease at diagnosis.
Despite high initial response rates to first-line chemotherapy (60-70%), nearly all patients relapse within months. Second-line treatment options are limited, with median overall survival of just 6 months for patients who respond to salvage therapy. The five-year survival rate for extensive-stage SCLC is less than 2%.
Phase 2 data for ifinatamab deruxtecan had shown a 48.2% objective response rate—remarkable for a population with few alternatives—and earned the drug FDA Breakthrough Therapy Designation in August 2025. However, even in the mid-stage trial, 4.4% of patient deaths were deemed treatment-related.
What's Next
Daiichi Sankyo initiated a voluntary pause in recruitment before the FDA imposed its partial clinical hold. Patients already enrolled may continue treatment, but no new participants will be recruited. The companies are working with the FDA and an independent data monitoring committee to review safety data.
"We are evaluating the potential impact of the partial clinical hold on study data readout timing, currently projected for fiscal year 2027," the companies said.
The hold does not affect I-DXd's other pivotal trials—IDeate-Prostate01 in metastatic castration-resistant prostate cancer and IDeate-Esophageal01 in advanced esophageal squamous cell carcinoma—though both indications also involve lung cancer-adjacent patient populations where ILD could prove problematic.
All eyes now turn to raludotatug deruxtecan (R-DXd), the third and final ADC in the Merck-Daiichi deal, which targets CDH6 and has shown impressive early data in ovarian cancer. However, ILD events have already been noted in its development program.
For Merck, which turned to Daiichi Sankyo after losing out to Pfizer in the $43 billion bidding war for Seagen, the setbacks raise questions about the company's ADC strategy and whether the deruxtecan payload's efficacy can ever be adequately balanced against its lung toxicity profile.
Related Companies: Merck & Co., Astrazeneca