Earnings summaries and quarterly performance for ASTRAZENECA.
Research analysts who have asked questions during ASTRAZENECA earnings calls.
Rajan Sharma
Goldman Sachs Group, Inc.
8 questions for AZN
Matthew Weston
UBS Group AG
7 questions for AZN
Mattias Häggblom
Handelsbanken
7 questions for AZN
Peter Verdult
Citigroup Inc.
7 questions for AZN
Justin Smith
Bernstein
6 questions for AZN
Luisa Hector
Berenberg
6 questions for AZN
Sachin Jain
Bank of America
6 questions for AZN
Simon Baker
Redburn Atlantic
6 questions for AZN
Steve Scala
Cowen
6 questions for AZN
Gonzalo Artiach
Danske Bank
5 questions for AZN
Seamus Fernandez
Guggenheim Partners
5 questions for AZN
James Gordon
JPMorgan Chase & Co.
4 questions for AZN
Richard Vosser
JPMorgan Chase & Co.
4 questions for AZN
Sarita Kapila
Morgan Stanley
4 questions for AZN
Michael Leuchten
Jefferies
3 questions for AZN
Rajesh Kumar
HSBC
3 questions for AZN
Christopher Uhde
SEB
2 questions for AZN
Graham Parry
Bank of America Corporation
2 questions for AZN
Mike Leuchten
Jefferies
2 questions for AZN
Emily Field
Barclays
1 question for AZN
Emmanuel Papadakis
Deutsche Bank
1 question for AZN
Eric Le Berrigaud
Stifel
1 question for AZN
Jo Walton
UBS
1 question for AZN
Mark Purcell
Morgan Stanley
1 question for AZN
Peter Welford
Jefferies
1 question for AZN
Richard Parkes
BNP Paribas Exane
1 question for AZN
Timothy Anderson
BofA Securities
1 question for AZN
Recent press releases and 8-K filings for AZN.
- AstraZeneca Finance LLC, with a full and unconditional guarantee from AstraZeneca PLC, issued $2.0 billion in aggregate principal amount of fixed rate notes.
- The issuance comprises $650,000,000 of 4.000% Fixed Rate Notes due 2031, $600,000,000 of 4.300% Fixed Rate Notes due 2033, and $750,000,000 of 4.600% Fixed Rate Notes due 2036.
- The settlement date for these notes is March 2, 2026.
- AstraZeneca's Calquence (acalabrutinib) in combination with venetoclax has been approved in the US by the FDA as the first all-oral, fixed-duration regimen for adult patients with chronic lymphocytic leukaemia (CLL) and small lymphocytic lymphoma (SLL).
- This approval is based on positive results from the AMPLIFY Phase III trial, which demonstrated that 77% of patients treated with the Calquence combination were progression-free at three years, significantly outperforming standard-of-care chemoimmunotherapy (67%).
- The Calquence combination offers a 14-month, fixed-duration treatment that reduced the risk of disease progression or death by 35% compared to chemoimmunotherapy.
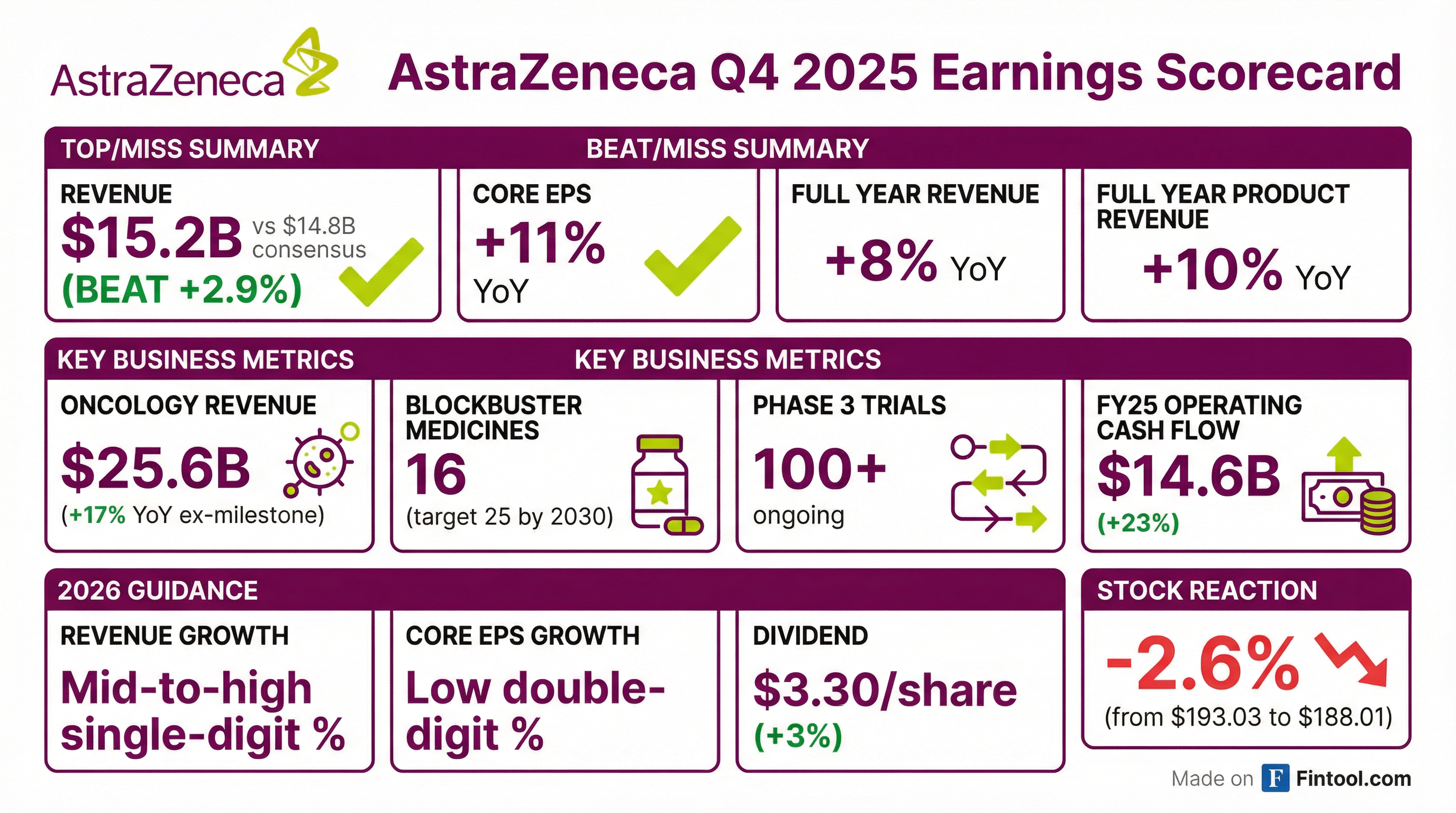
- AstraZeneca reported FY 2025 Total Revenue of $58,739 million, an increase of 9% (8% at CER), and Core EPS of $9.16, up 12% (11% at CER).
- For Q4 2025, Total Revenue was $15,503 million, up 4% (2% at CER), and Core EPS was $2.12, up 1% (down 2% at CER).
- The company provided FY 2026 guidance, expecting Total Revenue to increase by a mid-to-high single-digit percentage and Core EPS to increase by a low double-digit percentage at constant exchange rates.
- A second interim dividend of $2.17 per share was declared, increasing the total dividend for FY 2025 by 3% to $3.20 per share, with an intention to further increase the annual dividend to $3.30 per share for FY 2026.
- AstraZeneca reported strong financial performance for 2025, with revenue growing 8% and core EPS growing 11%, driven by continued global demand for its innovative medicines.
- For 2026, the company anticipates total revenue to grow by a mid- to high-single-digit percentage and core EPS growth of a low double-digit percentage at constant exchange rates.
- The company declared a full-year 2025 dividend of $3.20 per share and intends to increase the annual declared dividend to $3.30 per share in 2026.
- AstraZeneca maintains a robust pipeline with over 100 Phase III trials ongoing and expects 20 Phase III readouts in 2026, which could collectively add over $10 billion in peak revenue potential. The company remains on track for its $80 billion revenue target by 2030.
- AstraZeneca reported strong financial performance for 2025, with total revenue growing 8% and core EPS increasing 11%.
- The oncology segment delivered $25.6 billion in revenues, a 14% increase, while BioPharmaceuticals grew 5% to $23 billion.
- For 2026, the company anticipates total revenue growth of a mid- to high-single-digit % and core EPS growth of low double-digit % at constant exchange rates.
- The pipeline is robust, with 16 blockbuster medicines in 2025, aiming for 25 by 2030, and 20 phase 3 readouts expected in 2026 that could collectively generate over $10 billion in peak revenue.
- AstraZeneca reported strong financial performance in 2025, with total revenue growing 8% and core EPS increasing by 11%.
- For 2026, the company anticipates total revenue growth in the mid- to high-single-digit % and core EPS growth in the low double-digit % at constant exchange rates, with an intended annual declared dividend increase to $3.30 per share.
- The company highlighted significant pipeline momentum, with 16 blockbuster medicines in 2025 and a target of 25 by 2030, expecting 20 phase 3 readouts in 2026 with a combined peak-year sales potential exceeding $10 billion.
- Despite anticipated headwinds like the loss of exclusivity for Farxiga in the US in April 2026, AstraZeneca remains confident in achieving its $80 billion revenue ambition by 2030.
- Capital allocation priorities remain unchanged, with a net debt-to-EBITDA ratio of 1.2 times and continued disciplined investment in R&D and manufacturing capacity for future growth.
- The US Food and Drug Administration (FDA) issued a Complete Response Letter (CRL) regarding the Biologics License Application (BLA) for Saphnelo (anifrolumab) for subcutaneous administration in adult patients with systemic lupus erythematosus (SLE).
- AstraZeneca has since provided the requested information to the FDA, with a decision on the updated application expected in the first half of 2026.
- While the US subcutaneous application is pending, intravenous (IV) Saphnelo remains commercially available, and the subcutaneous formulation was approved in the European Union (EU) in December 2025.
- AstraZeneca will pay Bristol-Myers Squibb (BMS) a mid-teens royalty for US sales of Saphnelo under an updated 2025 agreement.
- AstraZeneca's Imfinzi (durvalumab) in combination with FLOT chemotherapy has been recommended for approval in the EU by the CHMP for adult patients with resectable, early-stage and locally advanced gastric and gastroesophageal junction (GEJ) cancers.
- This recommendation is based on the MATTERHORN Phase III trial, which demonstrated a 29% reduction in the risk of disease progression, recurrence or death and a 22% reduction in the risk of death for the Imfinzi regimen compared to chemotherapy alone.
- If approved, this would be the first immunotherapy-based perioperative therapy for patients in this setting in the EU.
- Imfinzi is already approved in the US and other countries for this same indication based on the MATTERHORN results.
- AstraZeneca commenced trading its ordinary shares on the New York Stock Exchange (NYSE) on February 2, 2026, to provide broader access for US investors.
- This move harmonizes the trading of AstraZeneca ordinary shares across the NYSE, London Stock Exchange (LSE), and Nasdaq Stockholm (STO) under the ticker symbol "AZN".
- The prior listing of American Depositary Shares and US dollar bonds on Nasdaq ceased on January 30, 2026.
- AstraZeneca aims to grow annual revenue to $80 billion and launch 20 new medicines by 2030.
- AstraZeneca has entered into a strategic collaboration agreement with CSPC Pharmaceuticals to enhance its weight management portfolio for obesity and type 2 diabetes.
- The agreement includes eight programmes, granting AstraZeneca exclusive global rights outside of China to CSPC's once-monthly injectable weight management portfolio, which features a clinical-ready asset, SYH2082.
- AstraZeneca will make an upfront payment of $1.2 billion to CSPC, with potential development and regulatory milestones of up to $3.5 billion across all programmes, in addition to commercialisation and sales milestones plus tiered royalties.
- The collaboration provides AstraZeneca access to CSPC's advanced AI-driven peptide drug discovery platform and proprietary LiquidGel once-monthly dosing platform technology.
- The transaction is expected to close in the second quarter of 2026, subject to customary closing conditions and regulatory clearances.
Quarterly earnings call transcripts for ASTRAZENECA.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more