Pfizer Licenses Novavax's Matrix-M Adjuvant for Up to $530M
January 20, 2026 · by Fintool Agent

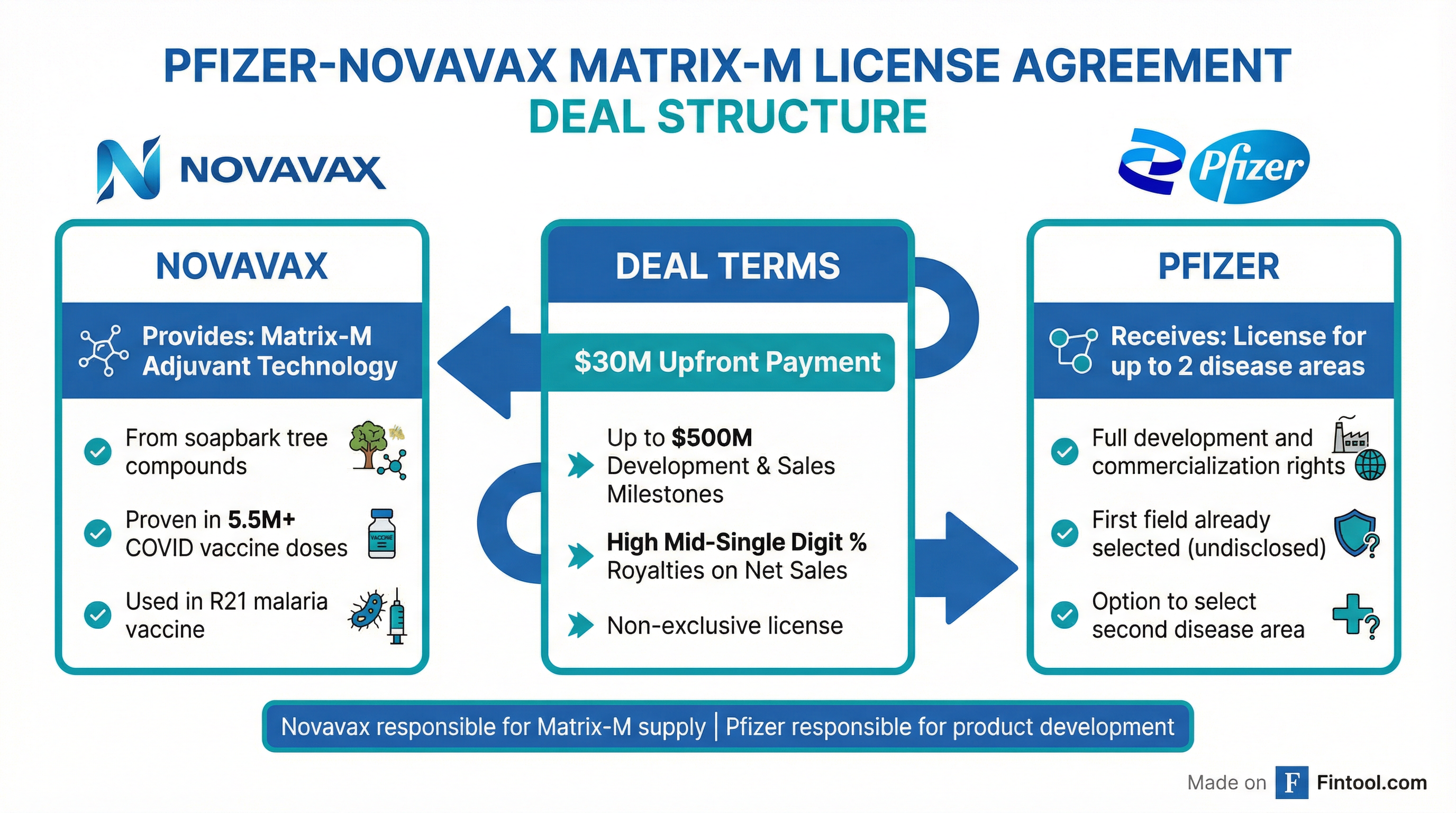
Pfizer is paying $30 million upfront to license Novavax's Matrix-M adjuvant technology, with potential milestone payments of up to $500 million, marking the second major pharma licensing deal for the vaccine-boosting platform and validating Novavax's pivot from COVID-19 vaccine maker to technology licensor.
The deal comes just two months after Pfizer divested over half its stake in pandemic vaccine partner BioNTech, signaling a strategic recalibration of its vaccine portfolio.
Deal Terms
Under the non-exclusive license agreement signed January 15, 2026:
- Upfront payment: $30 million to Novavax
- Development and sales milestones: Up to $500 million
- Royalties: Tiered high mid-single digit percentage on net sales
- Scope: License for up to two infectious disease areas
- Responsibilities: Pfizer handles development and commercialization; Novavax supplies Matrix-M
"The Novavax team is excited about this agreement with Pfizer to access our Matrix-M technology in its future development plans," said Novavax CEO John C. Jacobs. "This agreement, along with other recently formed partnerships, is further evidence of the potential utility of Matrix-M for the development of new products."
What is Matrix-M?
Matrix-M is Novavax's proprietary adjuvant technology derived from naturally occurring compounds in the bark of the Quillaja saponaria (soapbark) tree. The technology enhances the body's immune response to vaccines, allowing for:
- Broader, more potent immunity: Helps induce stronger and longer-lasting immune responses
- Lower antigen doses: Improves tolerability while reducing manufacturing costs
- Platform versatility: Compatible with multiple vaccine development approaches
The technology has a proven track record:
| Application | Status | Doses Administered |
|---|---|---|
| COVID-19 Vaccine (Nuvaxovid) | Authorized | 5.5+ million globally |
| R21/Matrix-M Malaria Vaccine | Authorized | 25 million distributed |
The malaria vaccine, developed with the University of Oxford and Serum Institute of India, represents a major public health achievement, providing protection against one of the world's deadliest diseases.
Pfizer's Vaccine Strategy Shift
The Novavax deal comes amid significant shifts in Pfizer's vaccine portfolio. In November 2025, the company sold more than half its stake in BioNTech—its mRNA vaccine partner responsible for Comirnaty, the COVID-19 vaccine that generated $37.8 billion in annual sales at its 2022 peak.
While Pfizer has maintained its operational collaboration with BioNTech remains unchanged, the divestiture and this new Matrix-M deal suggest a diversification strategy beyond mRNA technology.
Pfizer's vaccine pipeline includes several programs where adjuvant technology could prove valuable:
- C. difficile vaccine: Second-generation candidate in Phase 2, with Phase 3 expected by late 2025
- Fourth-generation PCV: Covers 25 serotypes with improved immunogenicity
- Fifth-generation PCV: In preclinical development covering 30+ serotypes
The specific disease areas Pfizer has selected for Matrix-M remain undisclosed, though the 8-K filing indicates the first field has already been chosen with an option to designate a second.
Novavax's Transformation
For Novavax, the Pfizer deal validates a dramatic strategic pivot. Once a $7+ billion market cap COVID-19 vaccine pure-play, the company has reimagined itself as a technology licensing platform following the pandemic's end.
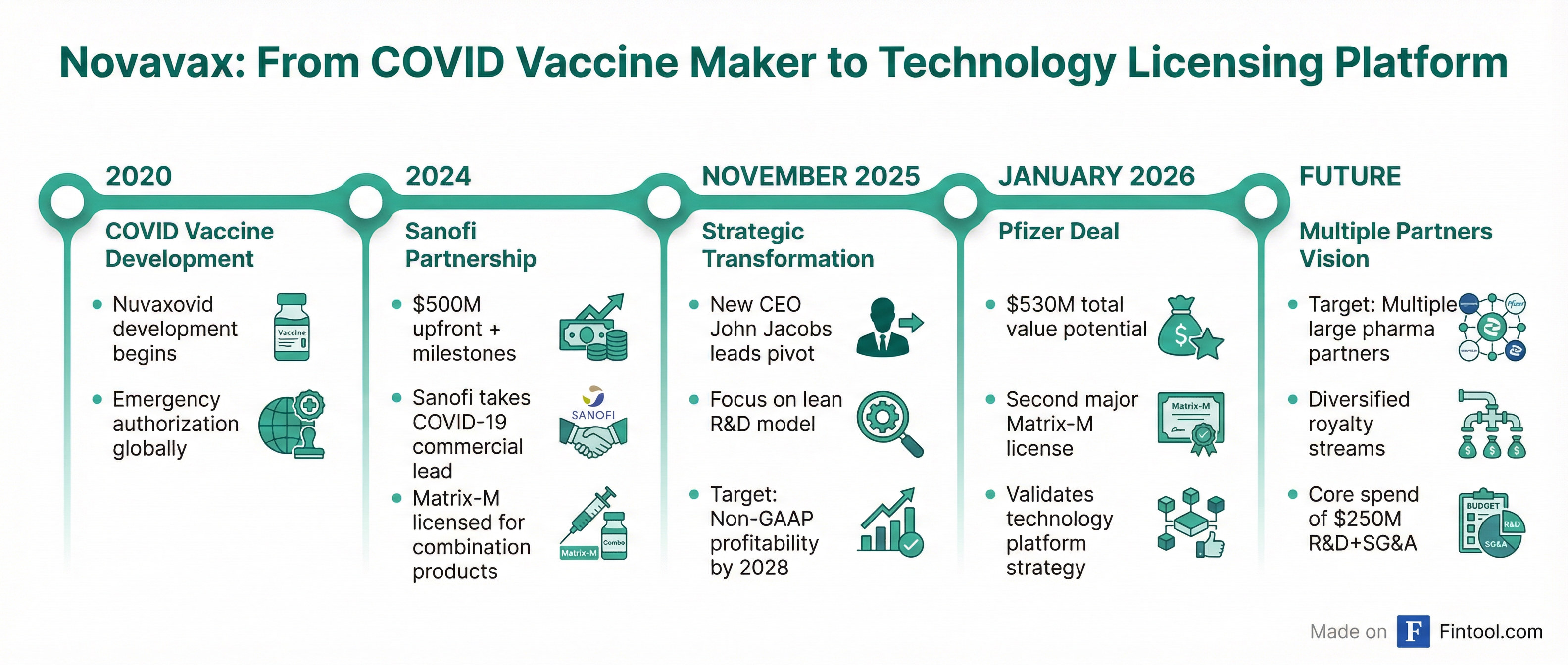
The transformation began with the landmark Sanofi partnership in 2024, which transferred COVID-19 commercial responsibilities and provided $500 million upfront plus milestone potential. Sanofi also licensed Matrix-M for combination products and its own vaccine development.
Novavax is now targeting a lean operating model with core R&D and SG&A spending of $250 million annually and aims for non-GAAP profitability as early as 2028.
| Period | Revenue | Net Income |
|---|---|---|
| Q1 2025 | $666.7M | $518.6M |
| Q2 2025 | $239.2M | $106.5M |
| Q3 2025 | $70.4M | ($202.4M) |
The revenue decline reflects the transition of COVID-19 commercial activities to Sanofi, but the company's licensing strategy is designed to create diversified, royalty-driven revenue streams from multiple partners.
Market Context
The deal arrives amid heightened uncertainty in the vaccine industry. Skepticism toward vaccines has grown under the Trump administration, with controversial policy discussions and rhetoric creating headwinds for the sector.
However, Sanofi CEO Paul Hudson suggested at the recent J.P. Morgan Healthcare Conference that long-term investors should view the environment as an opportunity. "If you're a short-term thinker, you don't move," he said. "If you're a long-term thinker—which is what we have to be—then there are less people to compete against to make acquisitions."
What to Watch
For Novavax (NVAX):
- Progress toward announced profitability target by 2028
- Additional Matrix-M licensing deals with other pharma partners
- Sanofi partnership milestones and royalty payments
- Q4 2025 earnings for full-year financial picture
For Pfizer (PFE):
- Disclosure of which disease areas Matrix-M will be applied to
- C. difficile vaccine Phase 3 initiation and data
- Progress on fourth-generation pneumococcal vaccine
- Any additional vaccine portfolio restructuring