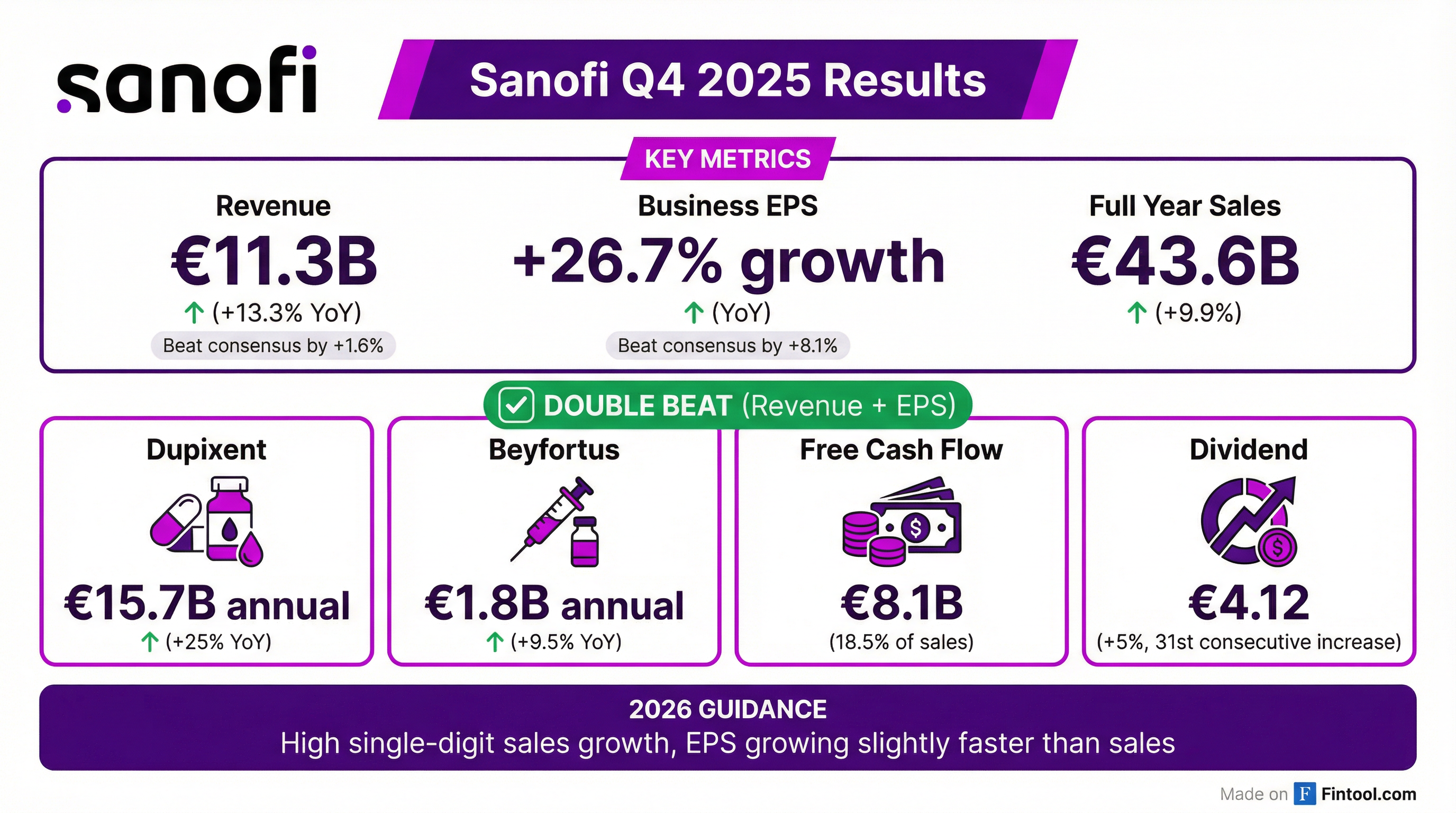
Earnings summaries and quarterly performance for Sanofi.
Research analysts who have asked questions during Sanofi earnings calls.
Peter Verdult
Citigroup Inc.
8 questions for SNY
David Risinger
Leerink Partners
7 questions for SNY
Luisa Hector
Berenberg
7 questions for SNY
Seamus Fernandez
Guggenheim Partners
7 questions for SNY
Simon Baker
Redburn Atlantic
7 questions for SNY
Florent Cespedes
Bernstein
6 questions for SNY
Ben Jackson
Jefferies
5 questions for SNY
Graham Parry
Bank of America Corporation
5 questions for SNY
James Quigley
Goldman Sachs
5 questions for SNY
Richard Vosser
JPMorgan Chase & Co.
5 questions for SNY
Sachin Jain
Bank of America
5 questions for SNY
Steve Scala
Cowen
5 questions for SNY
Sarita Kapila
Morgan Stanley
4 questions for SNY
Jo Walton
UBS
3 questions for SNY
Matthew Weston
UBS Group AG
3 questions for SNY
Eric Le Berrigaud
Stifel
2 questions for SNY
James Gordon
JPMorgan Chase & Co.
2 questions for SNY
Michael Leuchten
Jefferies
2 questions for SNY
Shirley Chen
Barclays
2 questions for SNY
Shirley Shin
Barclays
2 questions for SNY
Zane Abraham
JPMorgan Chase & Co.
2 questions for SNY
Colleen Garvey
Guggenheim Securities
1 question for SNY
Emily Field
Barclays
1 question for SNY
Emmanuel Papadakis
Deutsche Bank
1 question for SNY
Gary Steventon
BNP Paribas Exane
1 question for SNY
Luisa Caroline Hector
Berenberg
1 question for SNY
Peter Welford
Jefferies
1 question for SNY
Ricardo Benevides Freitas
Santander
1 question for SNY
Timothy Anderson
BofA Securities
1 question for SNY
Xiaobin Gao
Barclays
1 question for SNY
Recent press releases and 8-K filings for SNY.
- The U.S. Food and Drug Administration (FDA) has approved dupilumab (Dupixent), co-developed by Sanofi and Regeneron, for the treatment of allergic fungal rhinosinusitis (AFRS) in adults and children aged 6 and older.
- This approval makes Dupixent the first and only medicine specifically approved for AFRS, expanding its total approved uses to nine indications driven by type 2 inflammation.
- The decision represents a commercial and clinical milestone for Sanofi, offering a non-surgical treatment option for a recurrent disease.
- Sanofi handles international marketing for Dupixent, while it is jointly marketed with Regeneron in the U.S..
- Sanofi and Regeneron Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has approved Dupixent® (dupilumab) for the treatment of adult and pediatric patients aged 6 years and older with allergic fungal rhinosinusitis (AFRS) who have a history of sino-nasal surgery.
- This approval makes Dupixent the first treatment specifically approved for AFRS and was granted under Priority Review.
- The approval is supported by the LIBERTY-AFRS-AIMS Phase 3 trial, which demonstrated a 92% reduction in the risk of systemic corticosteroid use and/or need of surgery over 52 weeks for Dupixent compared to placebo.
- Dupixent is now approved in the U.S. for nine distinct diseases driven by type 2 inflammation, and over 1,400,000 patients are being treated globally across various indications.
- Sanofi's investigational drug, rilzabrutinib, has been granted Breakthrough Therapy designation by the US FDA and orphan designation in Japan for the treatment of warm autoimmune hemolytic anemia (wAIHA).
- These designations are based on clinical data from the ongoing LUMINA 2 phase 2b study and aim to expedite the development and review of rilzabrutinib for wAIHA, a rare autoimmune disorder with no specifically approved treatments.
- Rilzabrutinib (Wayrilz) is currently approved in the US, the EU, and the UAE for the treatment of adults with immune thrombocytopenia (ITP), and is under regulatory review for ITP in Japan.
- Sanofi and Teva Pharmaceuticals announced positive results from the RELIEVE UCCD long-term extension (LTE) study of duvakitug, an investigational human monoclonal antibody for ulcerative colitis (UC) and Crohn’s disease (CD).
- The study demonstrated durable clinical and endoscopic efficacy maintained over 44 weeks in patients who initially responded to the induction phase, with duvakitug being well tolerated.
- In UC patients, 58% (900 mg) and 47% (450 mg) achieved clinical remission at week 44 of the maintenance period.
- In CD patients, 55% (900 mg) and 41% (450 mg) achieved endoscopic response at week 44 of the maintenance period.
- Duvakitug is currently in phase 3 clinical studies for UC and CD, with Sanofi leading the development program. Teva will host an investor call and live webcast today, February 17, 2026, at 8:00 a.m. ET to discuss these data.
- Sanofi's Board of Directors decided not to renew the Director mandate of Paul Hudson, whose last day as Chief Executive Officer will be February 17, 2026.
- Belén Garijo has been appointed as the new Chief Executive Officer, taking up her duties on April 29, 2026, following the Group's Annual General Meeting.
- Olivier Charmeil will assume the role of Interim Chief Executive Officer during this transition period.
- Belén Garijo's priority will be to strengthen the productivity, governance, and innovation capacity of Research & Development.
- Sanofi is replacing CEO Paul Hudson, whose last day is February 17, 2026, with Belén Garijo set to take over after the April 29, 2026 Annual General Meeting (AGM).
- Olivier Charmeil will serve as interim CEO during the transition period.
- The board's decision is aimed at accelerating execution and bolstering R&D productivity, governance, and innovation.
- Hudson's departure follows a costly research push that failed to quickly replace sales threatened by a Dupixent patent cliff and mixed or negative results from several late-stage programs.
- The global asthma drugs market is projected to grow from USD 26.52 billion in 2025 to USD 41.18 billion by 2035, exhibiting a CAGR of 4.50% during the forecast period.
- Sanofi's Dupixent generated substantial revenue, with EUR 13,072 million (approximately USD 14,500 million) in sales for the full year 2024 and EUR 3,458 million (USD 3,800 million) in Q4 2024.
- The market is rapidly shifting towards high-value biologic therapies, with significant revenues reported by top players like Sanofi, Amgen, AstraZeneca, and GSK.
- Companies are making heavy R&D investments, with Sanofi allocating EUR 606 million for pharma launches and R&D in Q1 2024, and a wave of new product launches anticipated between 2025 and 2027.
- The global smart inhaler market was valued at USD 1.53 billion in 2024 and is projected to reach USD 7.9 billion by 2030, indicating a growing digital health segment.
- Sanofi has completed the acquisition of Dynavax Technologies Corporation on February 10, 2026.
- The acquisition integrates Dynavax's adult hepatitis B vaccine HEPLISAV-B and its shingles vaccine candidate (Z-1018) into Sanofi's portfolio, enhancing its adult immunization presence.
- Dynavax is now an indirect, wholly owned subsidiary of Sanofi, with untendered shares converted to $15.50 per share in cash.
- Dynavax common stock will cease to be traded on the NASDAQ Global Select Stock Market as of February 10, 2026.
- Sanofi's rilzabrutinib (Wayrilz) has been granted Breakthrough Therapy designation by the US FDA and Orphan Drug designation in Japan for the treatment of warm autoimmune hemolytic anemia (wAIHA).
- This marks rilzabrutinib as the first and only investigational Bruton’s tyrosine kinase (BTK) inhibitor for wAIHA to receive the FDA's Breakthrough Therapy designation.
- These designations are supported by clinical data from the ongoing LUMINA 2 phase 2b study, with a new LUMINA 3 phase 3 study also underway for wAIHA.
- The recognitions highlight the critical unmet medical need for wAIHA, a rare autoimmune disorder currently lacking a specific approved treatment.
- The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion recommending conditional marketing authorisation for Sanofi’s Rezurock (belumosudil) in the EU.
- This recommendation is for the treatment of adults and children aged 12 years and older with chronic graft-versus-host disease (GvHD), to be used when other treatment options are limited, unsuitable, or exhausted.
- The positive opinion follows a re-examination of a prior negative opinion adopted by the CHMP in October 2025, with a final European Commission decision expected in the coming weeks.
- Rezurock is already approved in 20 countries, including the US, UK, and Canada, and has treated over 17,000 patients since its first approval in the US in July 2021.
Quarterly earnings call transcripts for Sanofi.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more