Roche Enters $150 Billion Obesity Drug Race With 22.5% Weight Loss Data
January 27, 2026 · by Fintool Agent

Roche is making its move in the obesity drug wars. The Swiss pharma reported Phase 2 results Tuesday showing its experimental shot CT-388 helped patients lose 22.5% of their body weight—putting it in the same efficacy ballpark as Eli Lilly's+1.27% blockbuster Zepbound and significantly above Novo Nordisk's+0.17% Wegovy.
The data validates Roche's $2.7 billion bet on Carmot Therapeutics in 2023 and sets up a Phase 3 program launching this quarter. With analysts projecting the obesity market to exceed $150 billion annually, a third major player entering the race has significant implications for Lilly and Novo—the duopoly that has dominated the space.
"The headline data released today puts CT-388 pretty much into the same efficacy ballpark as Zepbound," Jefferies analysts wrote in a Tuesday note.
The Numbers That Matter
Roche's Phase 2 trial enrolled 469 people with obesity across five dosing cohorts. At the highest 24 mg dose after 48 weeks:
| Metric | Result |
|---|---|
| Placebo-adjusted weight loss (efficacy estimand) | 22.5% |
| Placebo-adjusted weight loss (treatment-regimen estimand) | 18.3% |
| Patients achieving ≥5% weight loss | 95.7% |
| Patients achieving ≥10% weight loss | 87.0% |
| Patients achieving ≥20% weight loss | 47.8% |
| Patients achieving ≥30% weight loss | 26.1% |
| Patients achieving BMI <30 (obesity resolution) | 54% vs 13% placebo |
| Discontinuation rate (drug-related) | 5.9% vs 1.3% placebo |
Perhaps most notable: Roche saw no plateau in weight loss at 48 weeks, suggesting longer treatment could yield even better results—a potential differentiator against competitors.
"To see no plateau and this very steep linear trajectory is likely a reflection of this potential for higher efficacy," Manu Chakravarthy, Roche's head of cardiovascular, renal, and metabolic development, told Reuters.
How CT-388 Stacks Up Against the Competition
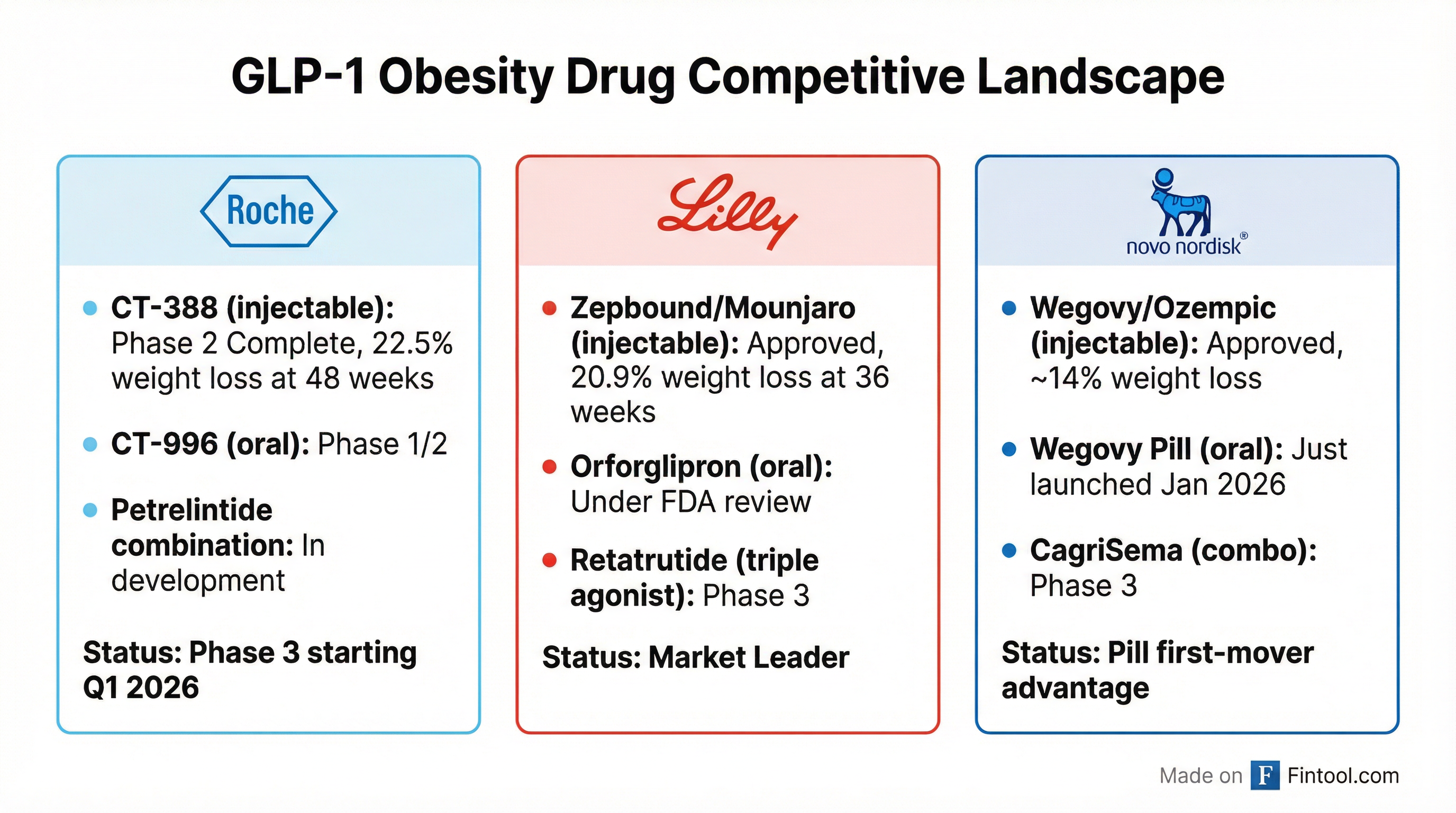
CT-388 is a dual GLP-1/GIP receptor agonist—the same drug class as Lilly's market-leading Zepbound (tirzepatide). Both work by targeting two hormonal pathways that regulate blood sugar and appetite. Novo Nordisk's Wegovy (semaglutide), by contrast, targets only GLP-1.
The efficacy data suggest CT-388 could be competitive:
| Drug | Company | Weight Loss | Trial Duration | Status |
|---|---|---|---|---|
| CT-388 | Roche | 22.5% | 48 weeks | Phase 3 Q1 2026 |
| Zepbound (tirzepatide) | Eli Lilly | 20.9% | 36 weeks | Approved 2023 |
| Wegovy (semaglutide) | Novo Nordisk | 14% | 68 weeks | Approved 2021 |
Direct cross-trial comparisons carry caveats—patient populations, endpoints, and methodologies differ. But the topline numbers position Roche to compete on efficacy.
The safety profile was "generally consistent with the incretin class," Roche said, with most gastrointestinal side effects mild to moderate. About 6% of patients discontinued due to adverse events, compared to 1.3% on placebo.
Roche's Obesity Strategy: Six Assets by 2030
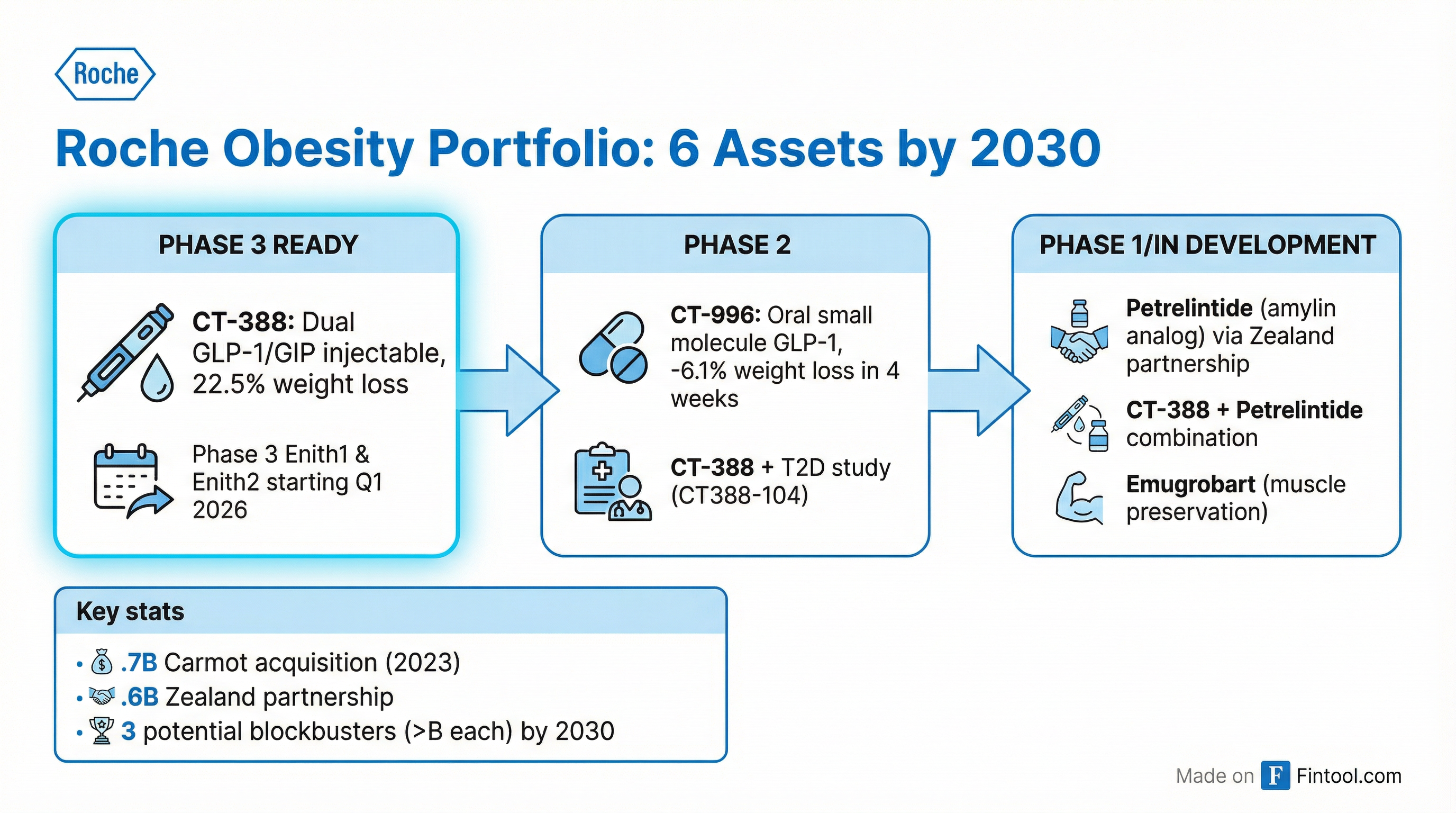
Roche isn't betting on a single drug. The company has built a portfolio of six obesity candidates through acquisitions and partnerships, targeting different mechanisms and patient populations.
Key pipeline assets:
- CT-388 (injectable): The lead asset entering Phase 3 this quarter with trials Enith1 and Enith2
- CT-996 (oral): A once-daily GLP-1 pill showing -6.1% weight loss in just 4 weeks of Phase 1 testing
- Petrelintide (amylin analog): Acquired via $1.6 billion Zealand Pharma partnership to address nausea
- CT-388 + Petrelintide combo: Potential best-in-class combination therapy
- Emugrobart: Targets muscle preservation during weight loss
Roche forecasts three of these could become blockbusters with annual sales exceeding $1 billion each by 2030.
"In fact, I would submit that if you only had [one megablockbuster], you would be severely handicapped in addressing the complexity and the heterogeneity of what we know the obesity market is," Chakravarthy said in September.
Why the Market Needed a Third Player
The obesity drug market has been a two-horse race between Lilly and Novo Nordisk, with combined GLP-1 sales expected to exceed $80 billion in 2026. Lilly's tirzepatide franchise (Mounjaro and Zepbound) is projected to hit $45 billion alone, while Novo's semaglutide products (Ozempic, Wegovy, Rybelsus) should reach $39.5 billion.
Both companies have struggled with supply constraints, creating openings for competitors. Roche's entry could:
- Pressure pricing: More competition typically drives prices down
- Expand supply: Additional manufacturing capacity
- Drive innovation: Competition accelerates R&D
"These first data are seen as positive, justifying the investments in the franchise," analysts at ODDO BHF wrote. "The combination of depth of response, absence of an early plateau and acceptable tolerability suggests CT-388 could sit in the top efficacy tier if replicated in Phase 3."
Market Reaction: Cautiously Optimistic
While the data are positive, investors appeared lukewarm. Roche shares traded roughly flat in Zurich, and analysts noted the company still faces a long road to market.
The skepticism reflects CT-388's timeline disadvantage. Even with successful Phase 3 trials, Roche likely won't reach market until 2028-2029—years behind Lilly and Novo. By then, both competitors will have expanded their portfolios with oral options (Novo's Wegovy pill launched earlier this month; Lilly's orforglipron is expected in 2026).
Jefferies analyst Michael Leuchten questioned whether CT-388 and CT-996 will be "meaningful sellers" given the competition. "They need to show a path to commercial relevance," he wrote.
What to Watch Next
Q1 2026: Enith1 and Enith2 Phase 3 trials initiate
2026: CT-996 oral GLP-1 advancing through trials
2027-2028: Phase 3 readouts expected
2028-2029: Potential FDA submission and approval
The obesity market opportunity is undeniable—by 2035, over 4 billion people (more than half the global population) are projected to live with excess weight or obesity. Whether Roche can carve out meaningful share against deeply entrenched competitors remains the $150 billion question.