Sanofi Pays $2.2B for Dynavax, Betting on Hepatitis B and Shingles Vaccines
December 24, 2025 · by Fintool Agent

Sanofi is acquiring Dynavax Technologies for $2.2 billion in cash, paying $15.50 per share—a 39% premium to Tuesday's close—as the French pharmaceutical giant pushes deeper into adult vaccines at a time when U.S. vaccination policy faces unprecedented upheaval.
DVAX shares surged 37.5% in premarket trading to $15.31, just under the offer price. The deal marks Sanofi's third major acquisition of 2025 and comes hours before markets close early for Christmas Eve.
Deal Structure
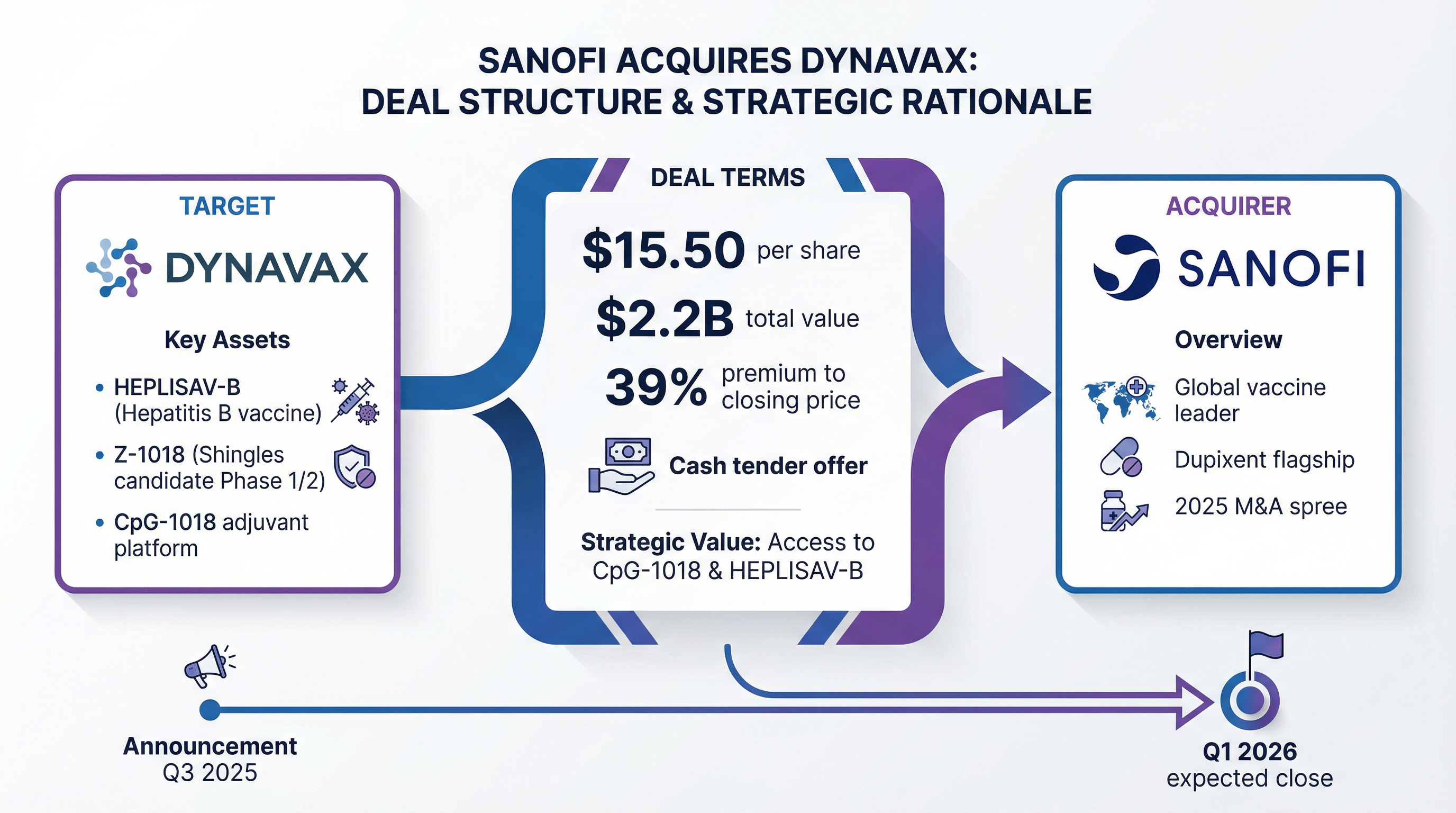
Sanofi will launch a cash tender offer for all outstanding Dynavax shares. The transaction has received unanimous board approval from Dynavax and is expected to close in Q1 2026, subject to HSR clearance and majority tender.
| Deal Metric | Value |
|---|---|
| Offer Price | $15.50/share |
| Total Equity Value | $2.2 billion |
| Premium to Close | 39% |
| Premium to 3-Month VWAP | 46% |
| Expected Close | Q1 2026 |
| Funding | Available cash |
Centerview Partners and Goldman Sachs advised Dynavax. Sanofi says the deal has no impact on 2025 guidance.
What Sanofi Gets
The acquisition centers on HEPLISAV-B, Dynavax's FDA-approved adult hepatitis B vaccine, which has carved out a dominant market position through a faster dosing schedule: two shots over one month versus the traditional three-dose, six-month regimen.
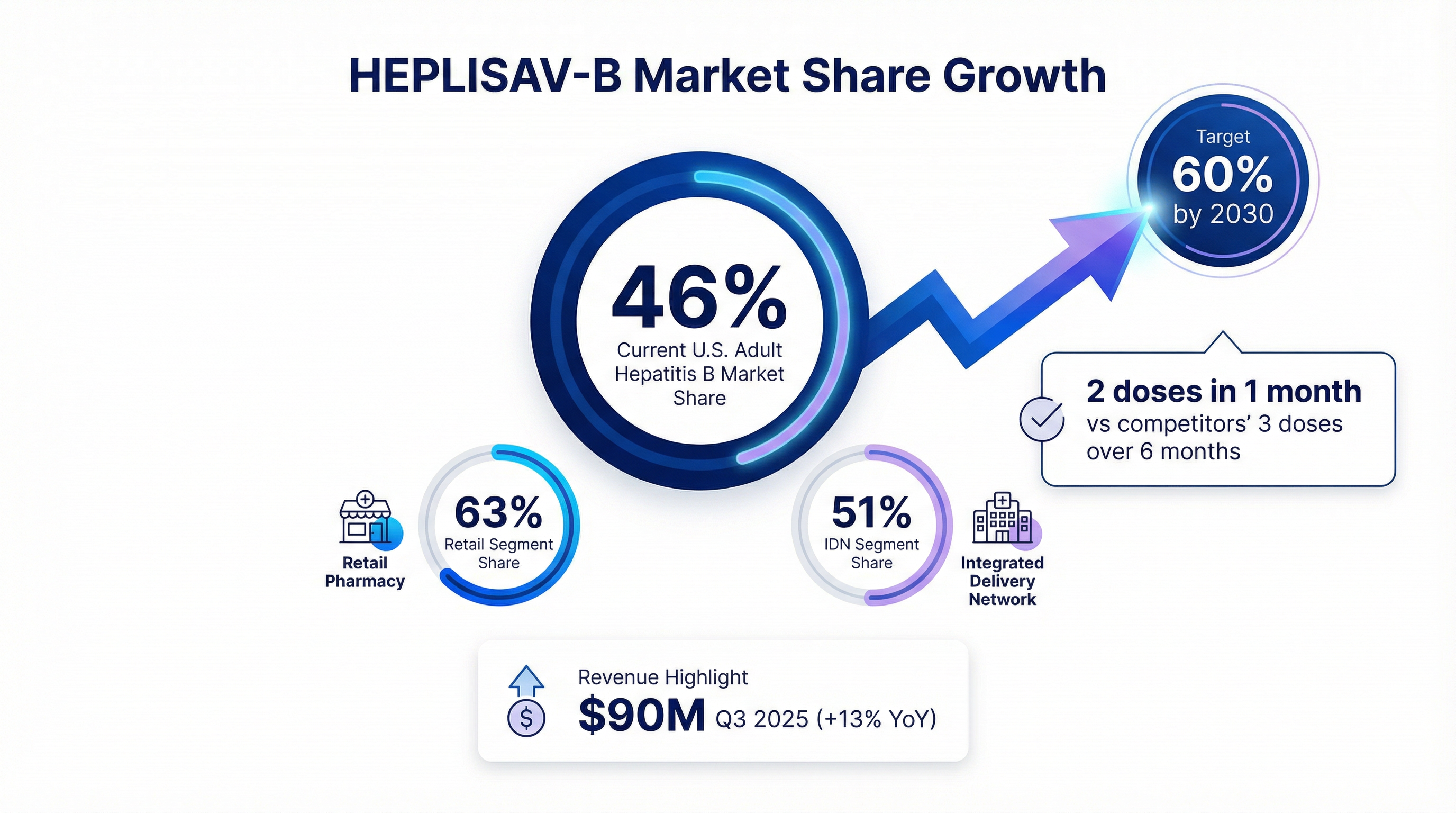
HEPLISAV-B posted $90 million in Q3 2025 revenue, up 13% year-over-year, with 46% U.S. market share—its highest ever. The retail pharmacy segment, which CEO Ryan Spencer expects to become "the dominant channel" by 2030, reached 63% share.
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenue | $71.1M | $65.0M | $91.9M | $90.0M |
| Net Income | $7.1M | -$96.1M | $18.7M | $26.9M |
| Gross Margin | 56.5%* | 52.2%* | 82.3%* | 65.3%* |
*Values retrieved from S&P Global
Dynavax projects the U.S. adult hepatitis B market will exceed $900 million by 2030, with HEPLISAV-B capturing at least 60% share. The massive catch-up opportunity: nearly 100 million American adults born before 1991 remain unvaccinated.
The Shingles Opportunity
Beyond hepatitis B, Sanofi gains Z-1018, an early-stage shingles vaccine that represents Dynavax's biggest pipeline bet.
Part 2 of the Phase 1/2 trial—focused on adults 70+ who face the highest shingles risk—began dosing in Q3 2025. Data from Part 1 showed CpG-1018 induced polyfunctional T cell responses "comparable to Shingrix," GSK's blockbuster. Top-line immunogenicity and safety results are expected in H2 2026.
The shingles market offers substantial prize money. GSK's Shingrix is on track for €4 billion in 2025 sales. J.P. Morgan analysts called the acquisition "a good fit," noting Z-1018's "potential for upside" if early data can be replicated.
Sanofi's 2025 M&A Spree
The Dynavax deal extends an aggressive acquisition strategy as Sanofi diversifies beyond Dupixent, its blockbuster asthma drug:
| Date | Target | Deal Value | Focus |
|---|---|---|---|
| June 2025 | BluePrint Medicines | Up to $9.5B | Rare diseases |
| July 2025 | Vicebio | $1.5B | RSV/respiratory vaccines |
| December 2025 | Dynavax | $2.2B | Adult vaccines |
"Dynavax enhances Sanofi's adult immunization presence by adding differentiated vaccines that complement Sanofi's expertise," said Thomas Triomphe, Sanofi's Executive VP of Vaccines.
The RFK Jr. Shadow
The deal arrives amid a challenging policy environment for vaccines. Health Secretary Robert F. Kennedy Jr. has targeted vaccination programs, cutting research funding and ousting the CDC director who oversees vaccine recommendations.
His advisory panel recently scrapped the long-standing recommendation that all American newborns receive hepatitis B shots—a decision that could theoretically narrow the eligible population for adult catch-up vaccination.
Earlier this year, Sanofi flagged lower vaccination rates partly due to "negative buzz" around vaccines. GSK has reported similar U.S. pressure, and Australia's CSL delayed plans to spin off its vaccine business citing "heightened volatility."
Yet Sanofi is doubling down. The bet: evidence-based vaccination will endure, and Dynavax's differentiated products—particularly the faster HEPLISAV-B regimen—can capture demand that persists.
Same-Day FDA Setback
In an ironic twist, Sanofi announced the Dynavax deal just minutes after disclosing that the FDA had rejected tolebrutinib, its experimental multiple sclerosis drug.
"We believe that the FDA should also take the advice of scientific experts, clinicians, and patients in this matter," said Houman Ashrafian, Sanofi's R&D chief. The rejection adds to disappointments in 2025 with eczema and smoker's lung candidates, contributing to Sanofi's underperformance versus European pharma peers.
Stock Performance
DVAX closed at $11.13 on December 23, having traded in a narrow $10.78–$11.29 range over the prior week. The $15.50 offer represents a 39% premium to that close and a 46% premium to the three-month volume-weighted average price.
The acquisition price values Dynavax at roughly 6.7x 2025 revenue (guidance midpoint of $320 million) and provides shareholders an immediate exit at levels not seen since early 2024.
Beyond the Core Assets
Dynavax brings additional pipeline assets to Sanofi:
COVID-19 oral vaccine: In November 2025, Dynavax licensed Vaxart's phase 2b oral COVID vaccine candidate, structured with just $25 million upfront and the right—but not obligation—to proceed after seeing data in late 2026.
Plague vaccine: Fully funded by the Department of Defense with $14 million in additional recent funding, currently in Phase 2.
Pandemic influenza: Phase 1/2 completed Part 1 with high seroconversion; advancing selected formulations to Part 2.
CpG-1018 adjuvant platform: Dynavax's proprietary toll-like receptor 9 agonist enhances immune response across vaccine candidates.
What to Watch
Q1 2026: Deal closing, subject to regulatory clearance and majority tender
H2 2026: Z-1018 shingles vaccine Phase 1/2 data readout—a key catalyst for valuing Sanofi's pipeline optionality
2026–2030: HEPLISAV-B market share trajectory toward 60% target as retail vaccination expands
Policy risk: Continued monitoring of RFK Jr.'s impact on vaccination rates and CDC recommendations
Related