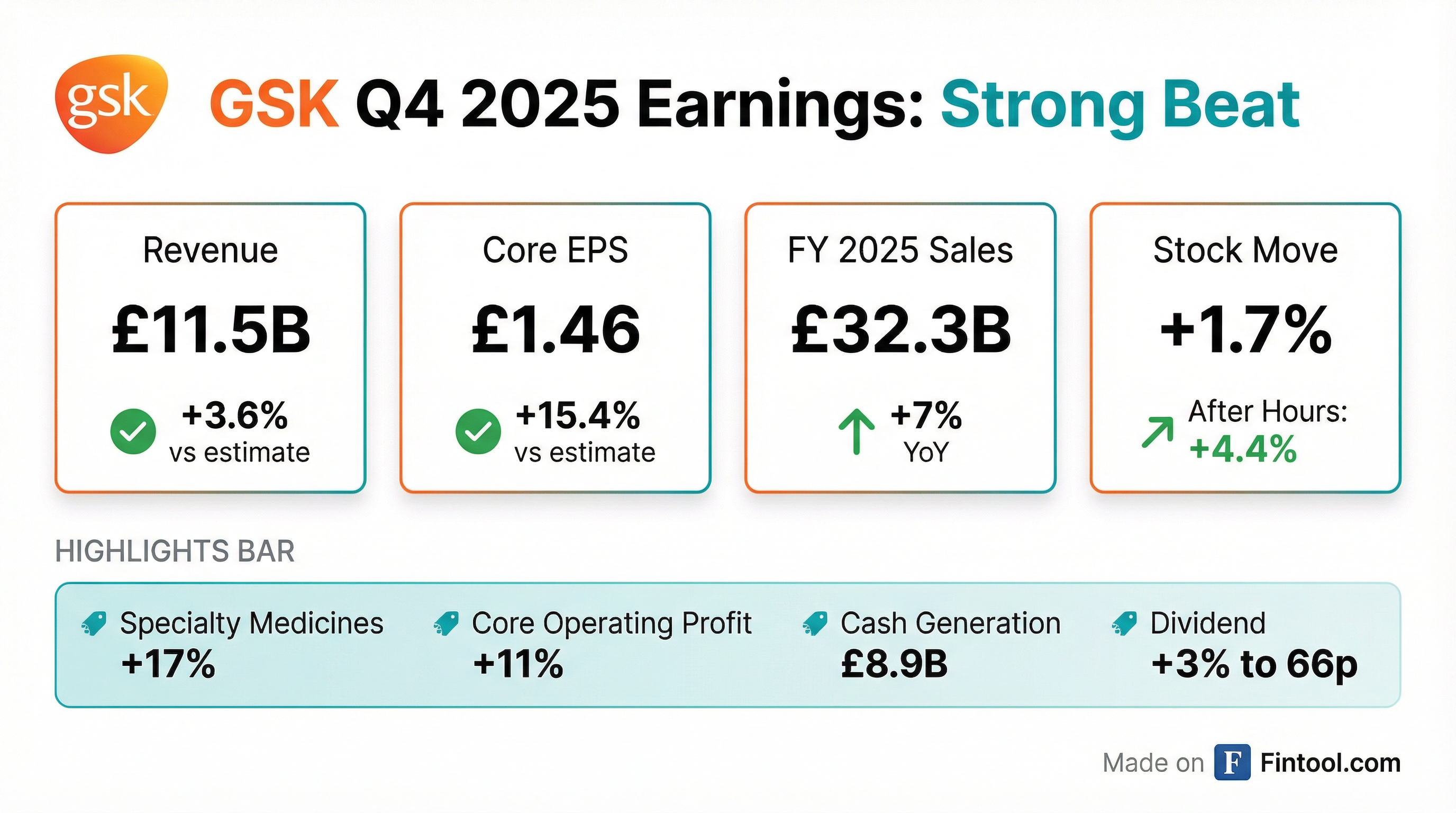
Earnings summaries and quarterly performance for GSK.
Research analysts who have asked questions during GSK earnings calls.
James Gordon
JPMorgan Chase & Co.
6 questions for GSK
Simon Baker
Redburn Atlantic
5 questions for GSK
Steve Scala
Cowen
4 questions for GSK
Graham Parry
Bank of America Corporation
3 questions for GSK
Michael Leuchten
Jefferies
3 questions for GSK
Rajan Sharma
Goldman Sachs Group, Inc.
3 questions for GSK
Sachin Jain
Bank of America
3 questions for GSK
Emily Field
Barclays
2 questions for GSK
Kerry Holford
Berenberg
2 questions for GSK
Peter Verdult
Citigroup Inc.
2 questions for GSK
Peter Welford
Jefferies
2 questions for GSK
Richard Parkes
BNP Paribas Exane
2 questions for GSK
Jo Walton
UBS
1 question for GSK
Justin Smith
Bernstein
1 question for GSK
Mark Purcell
Morgan Stanley
1 question for GSK
Matthew Weston
UBS Group AG
1 question for GSK
Sarita Kapila
Morgan Stanley
1 question for GSK
Timothy Anderson
BofA Securities
1 question for GSK
Recent press releases and 8-K filings for GSK.
- GSK plc purchased 516,000 ordinary shares on March 9, 2026, through BNP Paribas SA, as part of its existing buyback programme.
- The volume-weighted average price paid per share was 2,028.51p, with the lowest price at 1,989.00p and the highest at 2,047.00p.
- Since February 17, 2026, the company has acquired a total of 7,672,000 ordinary shares.
- Following this purchase, GSK plc holds 247,563,094 ordinary shares in treasury, representing 6.08% of the total voting rights.
- GSK plc has entered into a license agreement with Alfasigma S.p.A., granting Alfasigma worldwide exclusive rights to develop, manufacture, and commercialize linerixibat, an investigational treatment for cholestatic pruritus in primary biliary cholangitis (PBC).
- GSK will receive an upfront payment of $300 million and an additional $100 million upon US FDA approval, which is expected by 24 March 2026.
- Further potential payments to GSK include $20 million upon EU and UK approval, up to $270 million in sales-based milestone payments, and tiered double-digit royalties on net sales worldwide.
- Linerixibat's marketing applications are currently under regulatory review in the US, EU, UK, China, and Canada.
- GSK plc, acting through BNP Paribas SA, purchased 690,000 ordinary shares on March 6, 2026.
- The volume-weighted average price paid per share was 2,048.95p.
- These shares will be held as Treasury shares and are part of an existing buyback programme announced on February 17, 2026.
- Since February 17, 2026, the company has purchased a total of 7,156,000 ordinary shares.
- Following this purchase, GSK plc will hold 247,047,094 ordinary shares in treasury, representing 6.07% of voting rights.
- GSK plc purchased 550,000 of its ordinary shares on March 4, 2026, as part of its existing buyback programme.
- The shares were acquired at prices ranging from 2,109.00p to 2,149.00p, with a volume-weighted average price of 2,129.91p.
- Since February 17, 2026, the company has purchased a total of 5,816,000 ordinary shares.
- Following this purchase, GSK plc holds 245,707,094 ordinary shares in treasury, which represents 6.04% of the total voting rights.
- GSK plc purchased 630,000 ordinary shares on 03 March 2026 at a volume-weighted average price of 2,139.22p.
- These shares will be held as Treasury shares and are part of an existing buyback programme.
- Since 17 February 2026, the company has purchased a total of 5,266,000 ordinary shares.
- Following this purchase, GSK holds 245,157,094 ordinary shares in treasury, representing 6.02% of voting rights.
- GSK plc has completed its acquisition of RAPT Therapeutics, a clinical-stage biopharmaceutical company focused on inflammatory and immunologic diseases.
- The acquisition includes ozureprubart, a long-acting anti-immunoglobulin E (IgE) monoclonal antibody currently in phase IIb clinical development for prophylactic protection against food allergens.
- The total cash consideration for this acquisition amounts to an approximate aggregate equity value of $2.2 billion, with GSK's net upfront investment being approximately $1.9 billion.
- GSK has obtained global rights to the ozureprubart program, excluding mainland China, Macau, Taiwan, and Hong Kong, and will be responsible for success-based milestone and royalty payments to RAPT's partner.
- Data from the phase IIb trial (prestIgE) assessing ozureprubart as monotherapy is expected in 2027, with phase III trials to follow.
- GSK plc, acting through BNP Paribas SA, purchased 500,000 of its ordinary shares on March 2, 2026.
- The shares were bought at prices ranging from 2,168.00p to 2,195.00p, with a volume-weighted average price of 2,180.14p.
- This transaction is part of an existing buyback programme, and since February 17, 2026, the company has purchased a total of 4,636,000 ordinary shares.
- Following this purchase, GSK plc holds 244,527,094 ordinary shares in treasury, which represents 6.01% of the total voting rights.
- GSK plc announced the purchase of 430,000 ordinary shares on February 27, 2026, as part of its existing buyback programme.
- The shares were acquired through BNP Paribas SA at a volume-weighted average price of 2,179.36p, with prices ranging from 2,153.00p to 2,197.00p.
- Since February 17, 2026, the company has purchased a total of 4,136,000 ordinary shares.
- Following this purchase, GSK holds 244,027,094 ordinary shares in treasury, representing 5.99% of the total voting rights.
- GSK announced that its new drug application for linerixibat has been accepted for priority review in China by the National Medical Products Administration for the treatment of cholestatic pruritus in patients with primary biliary cholangitis (PBC).
- The application is based on positive data from the GLISTEN phase III trial, which demonstrated a significant and sustained improvement in cholestatic pruritus and itch-related sleep interference versus placebo.
- Linerixibat, an investigational ileal bile acid transporter (IBAT) inhibitor, has also been granted Orphan Drug Designation in the US, EU, and Japan for this condition.
- Marketing applications for linerixibat are currently under Health Authority review in the US, EU, UK, and Canada, but the drug is not yet approved anywhere globally.
- GSK announced that Japan's Ministry of Health, Labour and Welfare (MHLW) has accepted for review a new drug application (NDA) for bepirovirsen, an investigational antisense oligonucleotide for the treatment of adults with chronic hepatitis B (CHB).
- This represents the first global regulatory filing for bepirovirsen, which received SENKU designation in Japan in August 2024 to facilitate an expedited regulatory review process.
- The submission is supported by positive results from the pivotal phase III B-Well 1 and B-Well 2 trials, which demonstrated statistically significant and clinically meaningful functional cure rates.
- Chronic hepatitis B is a significant public health issue, affecting nearly 1 million people in Japan and over 250 million people worldwide, with current standard of care offering low functional cure rates.
Fintool News
In-depth analysis and coverage of GSK.
Quarterly earnings call transcripts for GSK.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more