Zenas BioPharma CEO Buys $1.6M in Stock After 52% Crash
January 11, 2026 · by Fintool Agent
Zenas Biopharma CEO Leon "Lonnie" Moulder Jr. purchased $1.63 million worth of company stock over three days last week, betting heavily on his own biotech after shares crashed more than 50% on Phase 3 trial results that disappointed Wall Street despite meeting all primary endpoints.
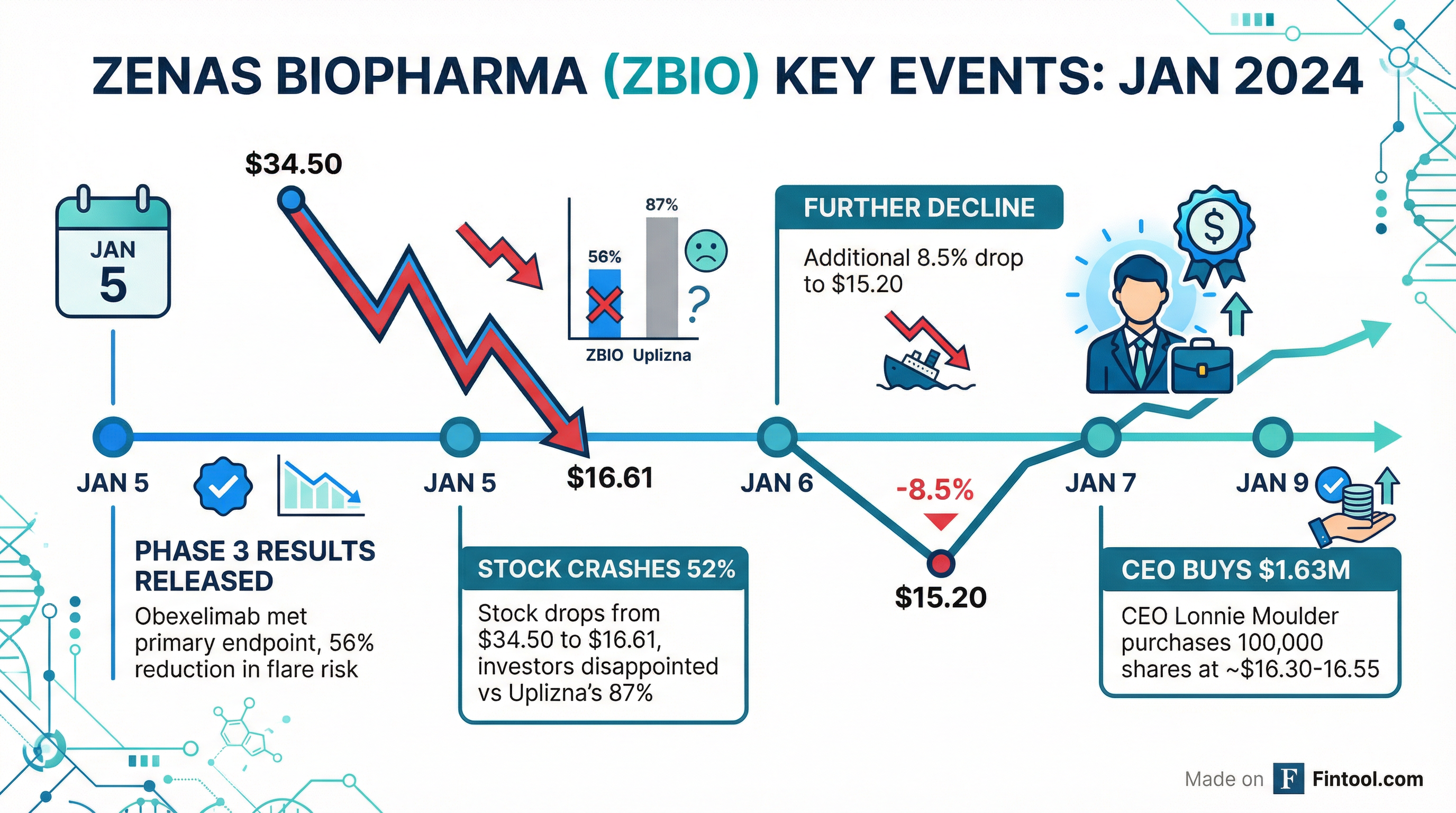
The serial biotech founder—who has led three companies to exits totaling over $9 billion—acquired 100,000 shares at prices ranging from $16.30 to $16.55 between January 7-9, according to SEC filings. The purchases came just days after ZBIO plunged from $34.50 to $16.61 in a single session.
The Crash: Winning the Trial, Losing the Market
On January 5, Zenas announced that its lead drug obexelimab met the primary endpoint in the Phase 3 INDIGO trial for IgG4-Related Disease (IgG4-RD), a rare autoimmune condition affecting multiple organs. The drug demonstrated a 56% reduction in disease flare risk compared to placebo (HR 0.44, p=0.0005)—a highly statistically significant result.
Obexelimab also hit all four secondary endpoints: reducing investigator-assessed flares, lowering the number of flares requiring rescue therapy, boosting complete remission rates, and cutting cumulative rescue therapy use. Safety data was favorable, with lower infection rates than placebo.
So why did the stock collapse?
Investors compared the results to Amgen's Uplizna (inebilizumab), which showed an 87% risk reduction in its own IgG4-RD trial. While the trials weren't head-to-head comparisons and enrolled different patient populations, the gap raised questions about obexelimab's commercial competitiveness.
"Disappointed that the hazard ratio doesn't hit a number that many people were hoping for," Moulder acknowledged on a January 6 conference call—a rare moment of candor from a CEO that sent shares down another 8.5% to $15.20.
A CEO With a Proven Track Record
What makes this insider buying particularly notable is Moulder's history. This isn't a first-time founder making a symbolic purchase—it's a serial biotech operator with a remarkable track record of creating shareholder value.
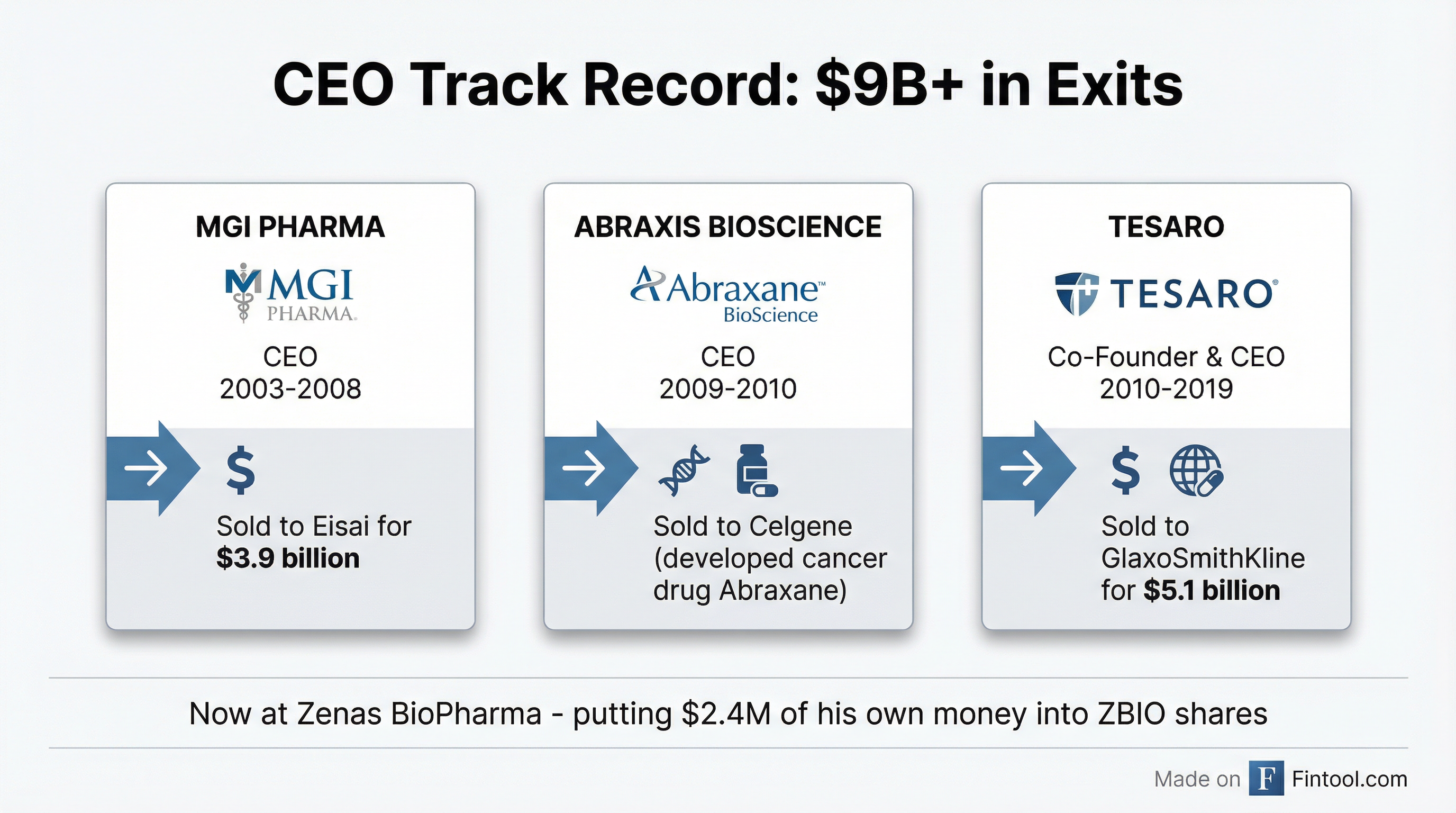
The Moulder resume:
- MGI PHARMA (CEO 2003-2008): Built the company from a small specialty pharma into an acquisition target. Sold to Japan's Eisai for $3.9 billion.
- Abraxis BioScience (CEO 2009-2010): Led the company that developed Abraxane, now a widely-used cancer treatment. Acquired by Celgene.
- TESARO (Co-Founder & CEO 2010-2019): Built the oncology-focused biotech from scratch. Sold to GlaxoSmithKline for $5.1 billion in January 2019. Named EY Entrepreneur of the Year 2017.
That's over $9 billion in aggregate exit value across three companies. Moulder, 67, holds degrees from Temple University (Pharmacy) and The University of Chicago Booth School of Business (MBA).
Insiders Are Unanimous: All Buys, No Sells
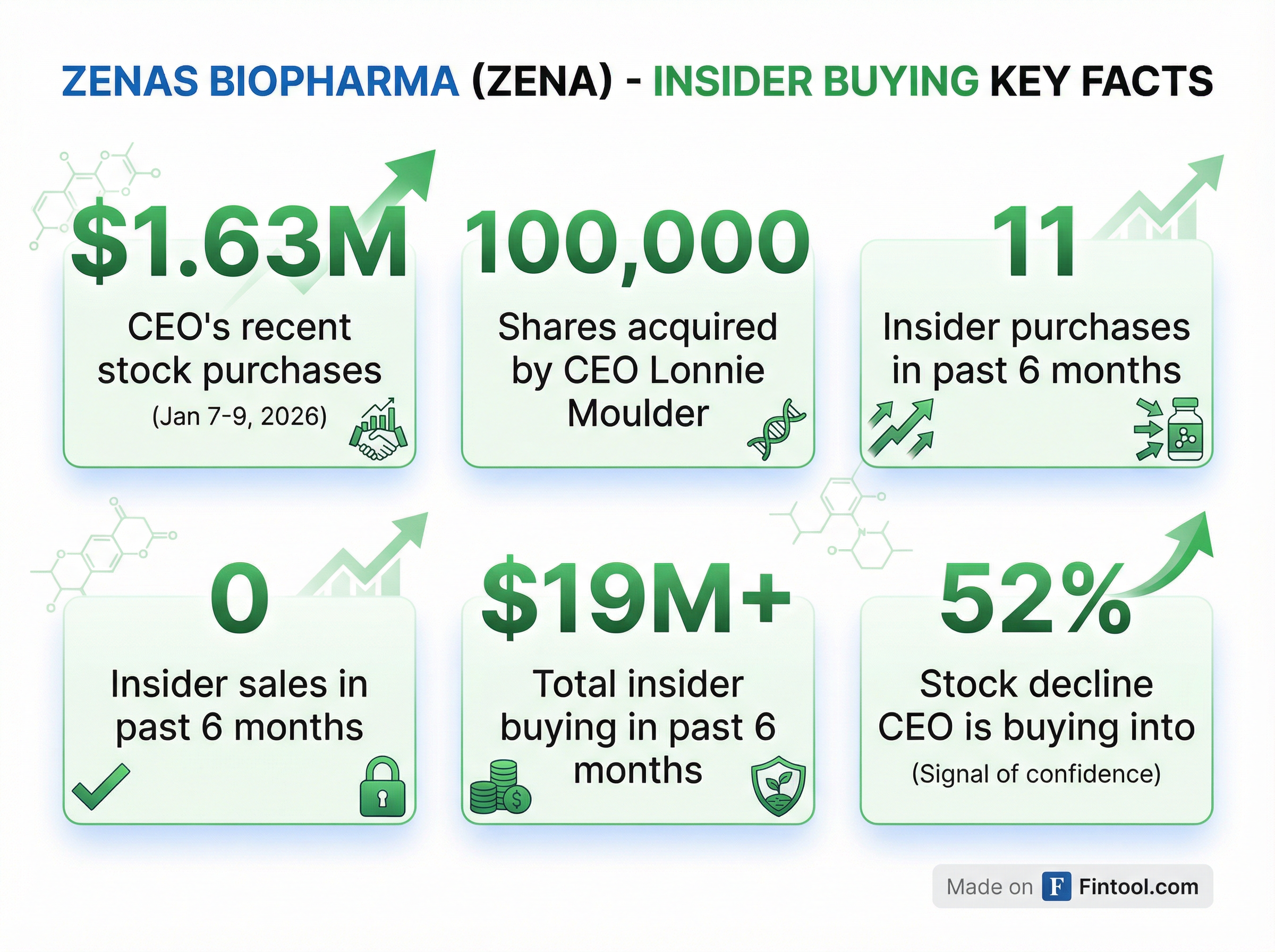
Moulder isn't alone. Over the past six months, Zenas insiders have made 11 open-market purchases totaling approximately $19 million—with zero sales.
Key insider buyers include:
- Leon O. Moulder Jr. (CEO): 4 purchases, $2.4 million, 136,928 shares
- Fairmount Funds Management: 316,219 shares, ~$6.0 million
- Hongbo Lu (Director): 263,160 shares, ~$5.0 million
- Patrick G. Enright (Director): 117,255 shares, ~$2.3 million
- SR One Capital Management: 126,315 shares, ~$2.4 million
The uniformity of insider buying—and complete absence of selling—stands out. In biotech, where executives often sell shares to diversify personal holdings, an 11-0 buy-to-sell ratio over six months sends a clear signal.
The Bull Case: Why the Market May Be Wrong
Despite the selloff, Zenas is proceeding with its regulatory plans. The company expects to submit a Biologics License Application (BLA) to the FDA in Q2 2026 and a Marketing Authorization Application to the EMA in H2 2026.
Several factors support the bull thesis:
Differentiated Mechanism: Unlike B cell-depleting agents like Uplizna, obexelimab inhibits B cell activity without depleting them. This preserves patients' ability to respond to vaccinations and infections—a meaningful advantage for a chronic disease requiring long-term treatment.
Subcutaneous Convenience: Obexelimab is a weekly self-administered subcutaneous injection, while Uplizna requires intravenous infusion at a healthcare facility. For a lifelong condition, at-home administration matters.
Pipeline Depth: Beyond IgG4-RD, Zenas has obexelimab in Phase 2 for Systemic Lupus Erythematosus (data expected Q4 2026) and multiple other programs including orelabrutinib for progressive multiple sclerosis.
Bristol Myers Squibb Partnership: Bristol Myers Squibb holds exclusive rights to obexelimab in Asia-Pacific markets including Japan, validating the drug's potential.
Analyst reactions have been mixed. H.C. Wainwright maintained a Buy rating with a $44 price target, emphasizing the "clinically meaningful results." Jefferies kept its Buy rating but cut the target from $62 to $48.
The Bear Case: Why Investors Sold
The counterargument is straightforward: in a competitive market, the best product usually wins.
With Uplizna already approved and showing 87% risk reduction versus obexelimab's 56%, physicians and payers may favor the competitor despite different mechanisms and administration routes. IgG4-RD is a rare disease with an estimated 20,000 diagnosed patients in the U.S.—a market that may not support two major drugs.
There's also a financing overhang. Under Zenas's agreement with Royalty Pharma, the company was eligible for a $75 million milestone payment tied to "defined success criteria" in the INDIGO trial. According to the 8-K filing, Zenas is "not currently eligible for the milestone payment" and is in "active discussions" with Royalty Pharma about the terms.
The usual ambulance chasers have arrived: Pomerantz LLP filed an investor alert on January 9, investigating potential securities law violations—a routine occurrence after any major biotech selloff.
What to Watch
Q2 2026: FDA BLA submission for obexelimab in IgG4-RD. Regulatory acceptance and review timeline will be key.
H2 2026: EMA submission in Europe; Phase 2 SLE data readout.
Stock Levels: ZBIO traded as high as $44.60 over the past 52 weeks before the crash. The current price of ~$16-17 represents a significant discount—either a buying opportunity or a fair re-rating depending on commercial prospects.
Royalty Pharma Negotiations: Resolution of the milestone payment dispute will signal how partners view the data.
For investors, the question is whether a CEO with $9 billion in exits knows something the market doesn't—or whether even smart money can be wrong. At minimum, Moulder's $1.63 million purchase suggests the founder believes the selloff was overdone.
His track record has earned the benefit of the doubt before.
Related: